Introduction
Relapsing polychondritis (RP) is a rare, immune-mediated multi-systemic disease characterized by inflammation of multiple cartilaginous tissues, particularly those in the ear, nose, tracheobronchial tree, and eye [
1,
2]. Although the pathogenesis of RP remains unclear, an immunological mechanism is considered to be crucial [
1]. Accordingly, nearly 30% of RP reportedly coexists with other autoimmune inflammatory disorders, including rheumatoid arthritis, systemic lupus erythematosus, and Sjogren's syndrome [
3]. However, the co-occurrence of RP and Crohn disease (CD) is rare, and the treatment strategy for such cases remains uncertain. Herein, we present a first report of co-existing RP and CD successfully treated with infliximab (IFX).
Go to :

Case
This study was approved by the Institutional Review Board of the Keimyung University Dongsan Hospital (IRB No: DSMC 2020-05-064). The patient provided written informed consent for publication of clinical details and images.
A 35-year-old woman with polyarthralgia and intermittent fever for a 2-month duration was referred to our rheumatology clinic. She presented with pain in multiple joints, including both hands, both wrists, right ankle, and both knees, and reported that the pain was most prominent in both metacarpophalangeal and proximal interphalangeal joints. The patient was not on any medication and had no prior illness. Laboratory tests revealed that C-reactive protein was elevated to 6.32 mg/dL (range, 0–0.5 mg/dL), erythrocyte sedimentation rate was increased to 120 mm/hr (range, women <25 mm/hr), hemoglobin was decreased to 10.4 g/dL (range, 11–15 g/dL). Her rheumatoid factor was marginally elevated to 25.2 IU/mL (range, ≤20 IU/mL); however, anti-cyclic citrullinated peptide antibody and antinuclear antibody were absent. All other biochemical parameters, leukocyte count, and electrolyte values were within normal limits. X-rays of multiple joints were normal without evidence of erosion.
On physical examination, she presented bilateral injected conjunctiva (
Fig. 1), as well as bilateral auricular redness and tenderness, with sparing of the earlobes (
Fig. 2). Ophthalmological examination revealed bilateral episcleritis. An auricular biopsy was performed, and various inflammatory cells were observed in the chondro-dermal junction (
Fig. 3). Based on auricular cartilage inflammation, ocular inflammation, and seronegative arthritis, she was diagnosed with RP according to the RP diagnostic criteria documented by Michet et al. [
4].
 | Fig. 1.Photo of patient's eye. Bilateral conjunctival injection is observed. Ophthalmologic examination presents corneal ulcer and iritis. 
|
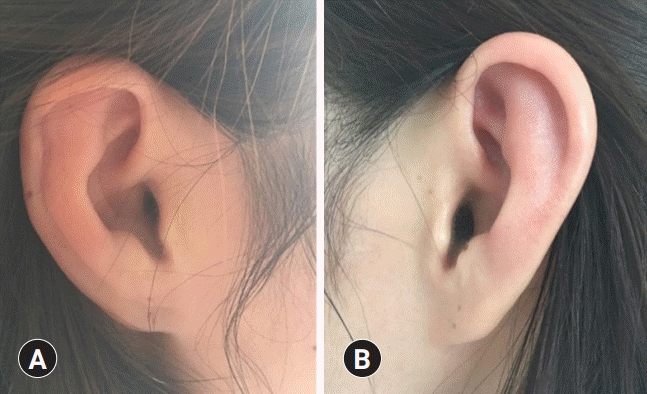 | Fig. 2.Photos of both ears. The bilateral erythema of both auricles with sparing of the ear lobes (A, right; B, left) indicates bilateral auricular chondritis. 
|
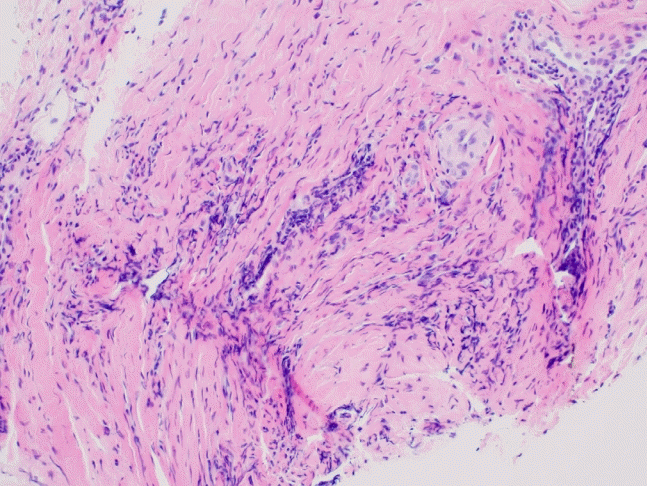 | Fig. 3.Microscopic finding of auricular biopsy. The microscopic finding of an auricular lesion shows perivascular infiltration of lymphocytes in a background of fibrous connective tissue (hematoxylin and eosin stain, ×200). 
|
Initial treatment was started with a low-dose steroid (prednisolone 10 mg) and non-steroidal anti-inflammatory drugs (NSAIDs) at the outpatient clinic. Three months later, she visited the emergency department with fever, abdominal pain, and intermittent hematochezia occurring for a month. A detailed interview focusing on gastrointestinal manifestations revealed that she had been experiencing intermittent vague abdominal pain and diarrhea 3–4 times per week for one year. She had self-diagnosed her symptoms as irritable bowel syndrome and had often consumed aloe vera as a folk remedy. Interestingly, she reported that her abdominal pain had repeatedly worsened, several times along with the exacerbation of RP-related symptoms, such as arthralgia, conjunctival injection, and bilateral auricular tenderness. Colonoscopy revealed multiple discontinuous ulcers with skipped lesions in the terminal ileum, cecum, and ascending colon, favoring a diagnosis of CD. Brownish, discolored colonic mucosa was observed, which was considered melanosis coli associated with the intake of aloe vera (
Fig. 4). Histological analyses of the colonoscopic biopsy revealed chronic inflammation. Her calprotectin level was >1,000 μg/g (range, <50 μg/g). Collectively, the patient was finally diagnosed with RP co-existing with CD.
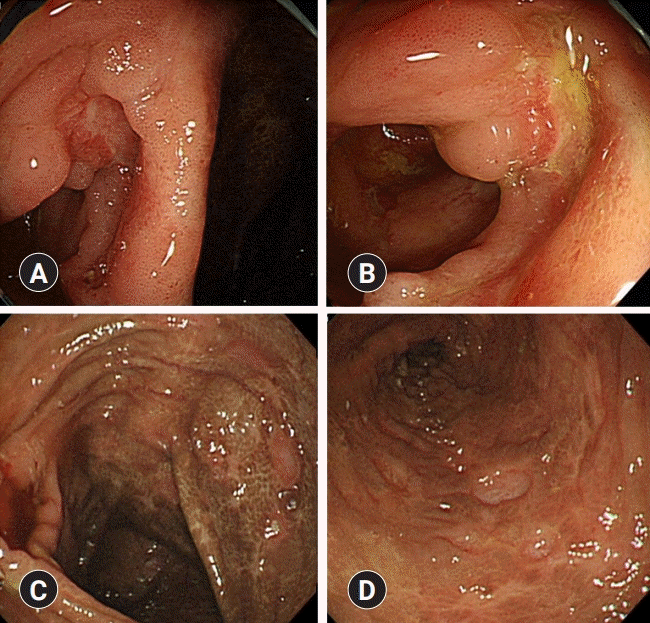 | Fig. 4.Colonoscopic findings. Multiple discontinuous ulcers are present in the terminal ileum (A, B), cecum, and ascending colon (C, D). Melanosis coli is observed probably due to the previous history of aloe vera consumption (C, D). 
|
After discontinuing NSAID therapy, she was treated with mesalazine (4 g/day), azathioprine (50 mg/day), and intravenous (IV) high-dose corticosteroid. With high-dose corticosteroid treatment, both CD- and RP-related symptoms were markedly improved. However, after tapering steroid treatment, she complained of mild, fluctuating, but persisting abdominal pain, diarrhea, bilateral auricular pain, and polyarthritis. Despite maintenance therapy comprising azathioprine and repeated steroid rescue therapy, flare-ups occurred twice in 6 months; thus, IV IFX (5 mg/kg) was initiated. Following induction therapy with IFX, all symptoms, including abdominal pain, diarrhea, auricular pain, and arthralgia, were improved. Eighteen months after treatment with IFX, the colonoscopy showed that only ulcer scars and a few inflammatory pseudopolyps remained. She had no RP recurrence and was confirmed to have reached not only a clinical but also an endoscopic remission with IFX.
Go to :

Discussion
RP is a long-lasting, systemic inflammatory disease presenting diverse clinical manifestations, with a flaring‒remitting course [
3]. As RP is an extremely rare disease, with an estimated prevalence of a few cases per million [
5], supporting data are extremely limited. To date, no specific laboratory or histological markers are available for RP, the diagnosis of RP is challenging and based on clinical findings [
1]. The criteria suggested by Michet et al. [
4] are the most commonly used for the diagnosis of RP and consist of the following: (A) two or more major criteria (auricular chondritis, nasal chondritis, laryngotracheal chondritis), (B) one or more of the previously mentioned major and two or more minor criteria (ocular inflammation, such as conjunctivitis, keratitis, episcleritis, and uveitis; hearing loss; vestibular dysfunction; seronegative inflammatory arthritis). The most frequent clinical feature of RP is auricular chondritis, causing unilateral or bilateral inflammation in the cartilage, with sparing of the earlobe. The second frequent manifestation is joint pain [
2]. These findings were unequivocally demonstrated in our current case. Regarding polyarthritis, our case differed from the clinical features of inflammatory bowel disease (IBD)-
related spondyloarthritis. There are two types of IBD-related peripheral arthritis: oligoarticular and polyarticular type [
6]. The oligoarticular type involves less than five joints, predominantly affecting the lower extremities. In contrast, the polyarticular type involves more than five small joints (predominantly upper limbs), with symmetric distribution and unrelated with IBD activity [
6,
7]. In this case, polyarthritis affecting both hands, both wrists, right ankle, and both knees, with episcleritis and unique auricular chondritis appeared simultaneously, which concurred with the criteria (B) of Michet et al. [
4].
Although the etiology of RP remains unclear, an autoimmune RP hypothesis is supported owing to its frequent association with other autoimmune diseases, as well as the effectiveness of corticosteroid and immunosuppressant treatments [
2]. As both RP and IBD are immune-mediated diseases, they can theoretically appear together. Reportedly, RP has been introduced as one of the extra-intestinal manifestations (EIMs) of IBD, but compared to other EIMs, such as peripheral and axial arthropathies, erythema nodosum, and pyoderma gangrenosum, RP is an extremely rare EIM of IBD [
8,
9]. Furthermore, RP is easily overlooked by clinicians, owing to its non-specific and unusual manifestations [
2]. However, proper diagnosis and treatment of RP are essential, as it can cause severe functional complications, including deafness, loss of vision, balance disturbance, and airway obstruction [
3,
10].
Owing to a lack of data, standardized RP treatment guidelines are yet to be established. Currently, the treatment strategy remains empirical, based on existing case reports. The treatment goal is symptomatic control and the long-term inhibition of immune-mediated pathogenic mechanisms [
1]. Thus, empirical tailored therapy, according to the severity of disease activity and organ involvement, has been provided [
10]. Mild forms of RP with the involvement of the external ear, nose, or joints are managed with NSAIDs, colchicine, dapsone, and low-dose corticosteroids, while severe RP forms, with organ-threatening complications, require high-dose corticosteroids [
2]. Immunomodulators, such as azathioprine, methotrexate, and cyclosporine, have been used as steroid-sparing therapy [
1]. Although evidence is extremely limited, biologics have been suggested as a secondary treatment for patients refractory to conventional therapies. According to a review of reports concerning RP treated with biologics, 62 patients with active RP were treated with anti-tumor necrosis factor alpha (anti-TNFα) agents; in 28, the treatment was effective, but six patients experienced only partial effects, and 28 reported no effect [
12]. In the IFX group, remission or partial remission was achieved in 18 of 31 patients [
11].
Although a few cases regarding the co-existence of RP and IBD have been documented, most have reported the co-existence of RP and ulcerative colitis, with the co-occurrence of CD extremely rare. To date, we have found only one case report in English through a PubMed search in March 2020; this patient showed resolution of RP with a mild form of CD after mesalazine treatment [
12]. In our case, a 35-year-old woman with RP and CD had recurring symptoms, with flare-ups following conventional treatment but responded well to IFX therapy. Following IFX treatment, both clinical and endoscopic remission was achieved and RP-related symptoms improved. No previous report has described the successful treatment of co-existing RP and CD with IFX.
In conclusion, clinicians should be aware that both RP and IBD can co-exist in patients. Additionally, anti-TNFα agents could be a treatment of choice for RP patients with IBD who are refractory to conventional immunosuppressive therapy.
Go to :


