Abstract
Donepezil is a cholinesterase inhibitor used extensively to treat Alzheimer disease. The increased cholinergic activity is associated with adverse effects, therefore gastrointestinal symptoms, including nausea, vomiting, and diarrhea, are common. Hypokalemia is a rare adverse event that occurs in less than 1% of donepezil-treated patients. Although hypokalemia of mild and moderate grade does not present serious signs and symptoms, severe hypokalemia often results in prolonged hospitalization and mortality. Herein, we report a case of hypokalemia developed after the initiation of donepezil therapy for cognitive impairment.
Go to : 
Hypokalemia is a common electrolyte disturbance in clinical practice. The major causes of hypokalemia include gastrointestinal loss and medications such as diuretics [1,2]. Most cases are asymptomatic and mild, but some patients develop severe hypokalemia resulting in arrhythmias and patient death. About 20% of inpatients experience hypokalemia during hospitalization [3], and among these cases, severe hypokalemia was sometimes associated with prolonged hospitalization and increased mortality [4].
Donepezil is the second approved acetylcholinesterase inhibitor for the treatment of mild to moderate Alzheimer disease by the United States Food and Drug Administration (FDA), extensively used worldwide [5]. As donepezil is generally tolerated, most adverse events are gastrointestinal symptoms, including vomiting and diarrhea [6]. However, rare adverse events such as lupus, psychosis, and arrhythmia have been reported in a few patients [7,8]. According to the FDA, hypokalemia is a rare adverse event that occurs in less than 1% of donepezil-treated patients [9,10].
Notably, hypokalemia exhibits non-specific symptoms such as general weakness, fatigue, dyspepsia, myalgia, tingling sensation, muscle cramps, and spasms; hence, it can be difficult to diagnose without laboratory investigations. Therefore, clinicians sometimes fail to recognize the signs and symptoms of hypokalemia. Accordingly, a rare case of hypokalemia induced by medication is significant. Herein, we report a case of hypokalemia developed after initiating donepezil as a treatment for cognitive impairment.
Go to : 
The study was approved by the Institutional Review Board of the Catholic University of Korea (IRB No: PC20ZASI0046) with waiver of informed consent.
An 87-year-old man visited the outpatient clinic owing to poor oral intake. According to his medical history, he had undergone surgery for benign prostatic hyperplasia 6 years ago and had discontinued the medication for benign prostatic hyperplasia 4 months before hospitalization. Recently, he was diagnosed with cognitive dysfunction based on a mini-mental status examination score of 22, and a global deterioration scale of 3. And was started on appropriate medication. At the time of admission, he was prescribed choline alfoscerate 400 mg twice daily and donepezil 5 mg for the control of Alzheimer disease.
A review of systems did not reveal abnormal findings. His vital signs were as follows: blood pressure (BP), 180/70 mmHg; pulse rate, 72 beats/min; respiratory rate, 20 breaths/min; body temperate, 36.7℃. To determine the cause of poor oral intake, routine investigations were performed, including complete blood count, blood chemistry, urine analysis, electrocardiogram, and chest X-ray. The presence of subtle pneumonia on the chest X-ray and hypokalemia (serum potassium 2.6 mmol/L) was detected. Initial serum inflammatory markers showed the following results: plasma leukocyte count, 7,500/mm3; serum c-reactive protein, 0.74 mg/dL (range, 0.01–0.5 mg/dL). Except for potassium, blood chemistry revealed the following: serum albumin, 4.0 g/dL (range, 3.5–5.2 g/dL); blood urea nitrogen, 10.9 mg/dL; serum creatinine, 0.72 mg/dL (range, 0.61–1.20 mg/dL); serum sodium, 140 mmol/L; serum chloride, 91 mmol/L; serum magnesium, 2.4 mg/dL; serum osmolality, 290 mOsm/kg. Urinalysis and urine sediment examination presented the following: urine pH, 7.5; urine specific gravity, 1.011; urine white blood cells, 0–2/high power field; urine red blood cells, 0–2/high power field. Blood gas analysis revealed compensated metabolic alkalosis as follows: arterial blood pH, 7.52; arterial blood pCO2, 46.6 mmHg; arterial blood pO2, 73.7 mmHg; arterial blood HCO3¯, 36.8 mmol/L. Chest X-ray and inflammatory markers suggested that the pneumonia was not severe. Therefore, hypokalemia could be the cause of underlying poor oral intake.
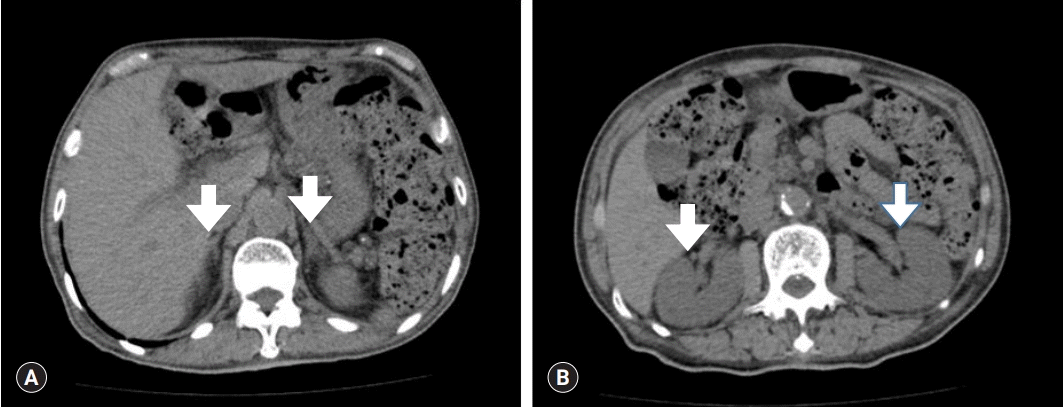
The patient was admitted to treat pneumonia and hypokalemia. For pneumonia treatment, he received ceftriaxone and clarithromycin as antibiotic agents. To determine the cause of hypokalemia, further investigations were performed. No clues indicating hypokalemia were detected in the medical history and review of systems. Next, his renal potassium excretion was measured to determine the potassium/creatinine ratio, fractional excretion of potassium, and transtubular potassium gradient (TTKG), using blood chemistry and spot urine chemistry. The results of spot urine chemistry were as follows: urea nitrogen, 392.8 mg/dL; creatinine, 90.8 mg/dL; total protein, 29.9 mg/dL; sodium, 35 mEq/L; potassium, 29.2 mEq/L; chloride, 45 mEq/L; osmolality, 309 mOsm/kg. Additionally, the following values were determined and suggested renal potassium wasting: urine potassium/creatinine ratio, 32.2 mEq/g; fractional excretion of potassium, 8.9%; TTKG, 10.5. Those findings suggested renal potassium wasting. Next, computed tomography demonstrated no abnormalities in the bilateral adrenal glands and both kidneys (Fig. 1). A hormone study was conducted to evaluate the cause of high BP. The thyroid function test was in the normal range. The adrenocorticotropic hormone (ACTH) level was 36.02 pg/mL (range, 10.0–60.0 pg/mL) and the cortisol level was 10.51 µg/dL (range, 9.41–26.06 µg/dL), which were within the normal range, revealing no mineral corticoid excess. Plasma renin activity was determined as 0.19 ng/mL/hr (range, 0.3–2.9 ng/mL/hr), aldosterone concentration was 14.93 pg/mL (range, 29.9–158.8 pg/mL), and the aldosterone/renin ratio was 7.86, indicating hyporeninemic hypoaldosteronism. However, unlike Liddle syndrome, the serum sodium level was normal at 140 mmol/L. The serum magnesium level was normal at 2.4 mg/dL, which was far from that observed in Gitelman syndrome. Hence, no obvious cause of hypokalemia was determined.
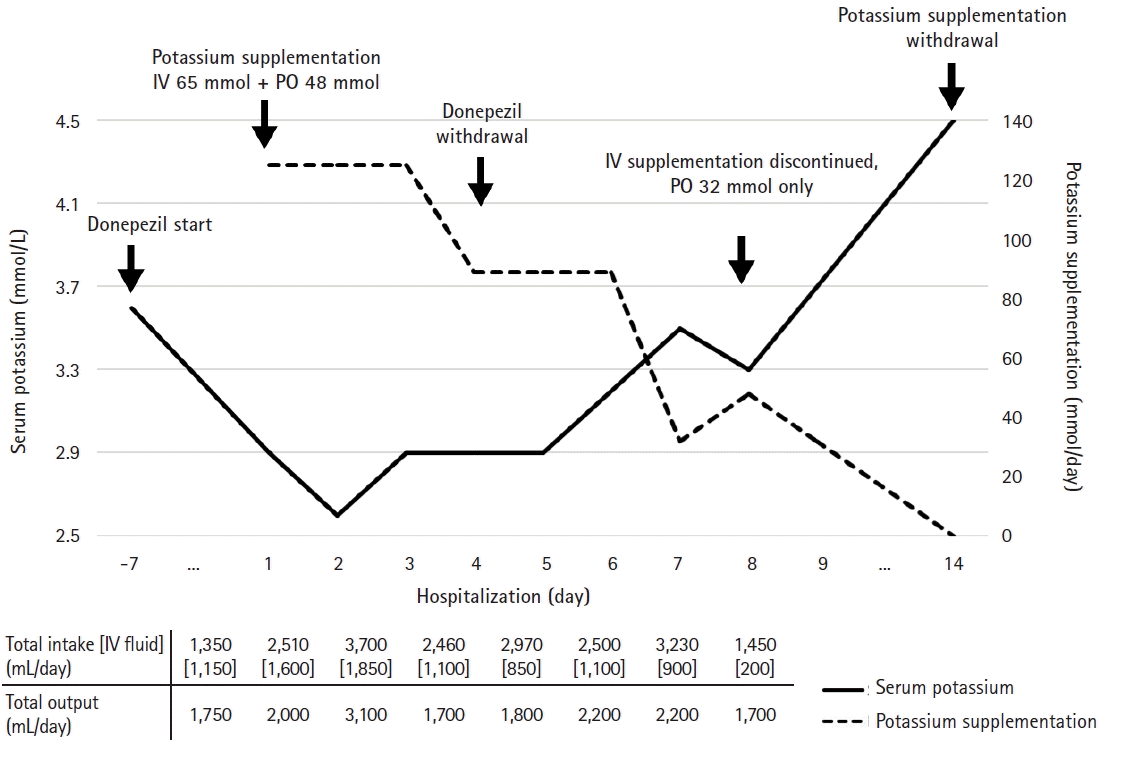
During investigations analyzing the possible cause of hypokalemia, he underwent potassium supplementation using intravenous and oral formulations. Until the fourth day of hospitalization, his serum potassium level demonstrated difficulty increasing beyond 3.0 mmol/L despite sufficient potassium administration (Fig. 2). As the hypokalemia was poorly corrected, clinicians suspected other causes of hypokalemia. A thorough review of the therapeutic agents prescribed for cognitive dysfunction was conducted. On examining possible adverse effects, we identified evidence suggesting that donepezil causes hypokalemia as a rare adverse event [9,10]. Therefore, donepezil was immediately discontinued. Thereafter, his potassium demand, supplemented intravenously, gradually decreased and was modified to oral potassium agents. On the third day after donepezil cessation, his serum potassium level recovered to 3.5 mmol/L under oral potassium supplementation of 32 mEq/day (Fig. 2). During hypokalemia evaluation and correction, pneumonia was properly controlled. Finally, he was able to maintain serum potassium of 3.3 mmol/L under oral potassium supplementation of 32 mEq/day and was discharged on the ninth day of hospitalization. At the time of discharge, the results of blood chemistry and spot urine chemistry were as follows: serum creatinine, 0.73 mg/dL; serum potassium, 3.5 mmol/L; serum magnesium, 2.3 mg/dL; serum osmolality, 295 mOsm/kg; urine creatinine, 120.6 mg/dL; potassium, 46.7 mEq/L; osmolality, 330 mOsm/kg. Additionally, the following values were determined: urine potassium/creatinine ratio, 38.7 mEq/g; fractional excretion of potassium, 8.1%; TTKG, 11.9.
Conversely, in the past, he had presented a systolic BP of less than 130 mmHg and diastolic BP of 80 mmHg during several visits to the outpatient clinic, with no history of medication-related hypertension. However, for 24 hours after hospitalization, BP was continuously confirmed as 160/90 mmHg or more, including a maximum of 185/105 mmHg, and amlodipine 5 mg was initiated. As BP measurements were above 140/90 mmHg on average after the addition of amlodipine 5 mg, the patient was additionally prescribed olmesartan 20 mg on the fifth day of hospitalization. Finally, in the outpatient clinic follow-ups, serum potassium was measured as 4.5 mmol/L, and oral potassium agents were withheld.
Go to : 
This case report presents the rare development of hypokalemia with donepezil, an agent used to treat cognitive dysfunction. The most common cause of hypokalemia is gastrointestinal losses, followed by medications such as diuretics [1,2]. Additionally, various conditions lead to hypokalemia. For example, the following factors result in the development of renal potassium wasting: malignant hypertension, renal artery stenosis, renin-secreting tumors that can increase renin, adrenal hyperplasia, Cushing syndrome, medication including diuretics, magnesium deficiency, Gitelman syndrome, and chronic metabolic acidosis [3]. However, it is difficult to implicate donepezil, used for cognitive dysfunction therapy, as a causative agent. If this patient had multiple prescriptions, donepezil was probably not considered a major cause of hypokalemia.
Donepezil is a cholinesterase inhibitor, mainly prescribed for Alzheimer disease. By inhibiting acetylcholinesterase, donepezil improves behavioral and cognitive symptoms, including confusion, aggression, and psychosis [11,12]. In several studies, donepezil has demonstrated improved cognitive functions in patients with dementia, but it had some adverse effects [13]. The adverse effects were associated with increased cholinergic activity, and the gastrointestinal system was mainly affected. Therefore, nausea, vomiting, and diarrhea were the most common symptoms, as well as insomnia, abnormal dreams, hepatotoxicity, and cardiovascular adverse events [14]. Hypokalemia was one of the rare adverse effects.
The mechanism by which donepezil causes hypokalemia remains unclear. In this case, based on TTKG, potassium excretion continued under donepezil therapy. Therefore, we hypothesized the mechanism by which donepezil caused renal potassium wasting. This finding may be due to the action of donepezil on the ion channels in the renal tubule or Henle's loop. Donepezil could potentially inhibit the Na+-K+-2Cl- cotransporter in the thick ascending limb of Henle's loop, suppressing potassium reabsorption. Additionally, it could be postulated that donepezil stimulates the renal outer medullary potassium channel to excrete potassium. As another hypothesis, donepezil could affect the sodium channel epithelium in the principal cell of the collecting tubule. Thereafter, potassium appeared to be secreted into the lumen to maintain electrolyte balance. Although the mechanism by which donepezil induces hypokalemia remains unclear, we anticipated that renal potassium wasting would recover after its withdrawal. Unfortunately, the follow-up study did not achieve demonstrate an improvement in renal potassium wasting. Based on previous reports, we speculated the possible reasons by which recovery from renal potassium wasting may vary from 2 days to 3 months [15,16]. Therefore, the patient may require further time for recovery. Nonetheless, this case was significant as it confirmed the progression of hypokalemia recovery, necessitating a decrease in the daily amount of potassium supplementation after the withdrawal of donepezil.
A limitation of this case report is that we have not done additional research regarding how donepezil affects the renal ion channels. Further case reports and research would assist in determining the mechanism by which donepezil causes renal potassium wasting. Although donepezil demonstrates rare serious adverse events, symptoms during early therapy require a differential diagnosis for hypokalemia.
Go to : 
Notes
Author contributions
Conceptualization: all authors; Data curation: DK, THB, HSP; Formal analysis: DK, THB, HEY, BSC, BSK; Investigation: DK, THB, HEY, HSP; Methodology, Project administration: THB; Supervision: THB, BSC, BSK; Validation: THB, SJS, BSC, BSK; Writing-original draft: DK; Writing-review & editing: DK, THB, HEY, HSP, SJS.
Go to : 
References
1. Crop MJ, Hoorn EJ, Lindemans J, Zietse R. Hypokalaemia and subsequent hyperkalaemia in hospitalized patients. Nephrol Dial Transplant. 2007; 22:3471–7.

2. Widodo D, Setiawan B, Chen K, Nainggolan L, Santoso WD. The prevalence of hypokalemia in hospitalized patients with infectious diseases problem at Cipto Mangunkusumo Hospital, Jakarta. Acta Med Indones. 2006; 38:202–5.
3. Unwin RJ, Luft FC, Shirley DG. Pathophysiology and management of hypokalemia: a clinical perspective. Nat Rev Nephrol. 2011; 7:75–84.

4. Paltiel O, Salakhov E, Ronen I, Berg D, Israeli A. Management of severe hypokalemia in hospitalized patients: a study of quality of care based on computerized databases. Arch Intern Med. 2001; 161:1089–95.

5. Li HC, Luo KX, Wang JS, Wang QX. Extrapyramidal side effect of donepezil hydrochloride in an elderly patient: a case report. Medicine (Baltimore). 2020; 99:e19443.
6. Arai H, Hashimoto N, Sumitomo K, Takase T, Ishii M. Disease state changes and safety of long-term donepezil hydrochloride administration in patients with Alzheimer's disease: Japan-Great Outcome of Long-term trial with Donepezil (J-GOLD). Psychogeriatrics. 2018; 18:402–11.

7. Cubeddu LX. Drug-induced inhibition and trafficking disruption of ion channels: pathogenesis of QT abnormalities and drug-induced fatal arrhythmias. Curr Cardiol Rev. 2016; 12:141–54.

8. Manzo C, Putignano S. Drug-induced lupus erythematosus associated with donepezil: a case report. Age Ageing. 2015; 44:1062–3.

9. US Food and Drug Administration (FDA). Highlights of prescribing information: Aricept [Internet]. Silver Spring (MD): FDA;2012. [cited 2020 Feb 20]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020690s035,021720s008,022568s005lbl.pdf.
10. eHealthMe. Will you have hypokalemia with Donepezil? (a study of FDA data) [Internet]. eHealthMe;2020. [cited 2020 Feb 20]. https://www.ehealthme.com/ds/donepezil/hypokalemia/.
11. Li XL, Hu N, Tan MS, Yu JT, Tan L. Behavioral and psychological symptoms in Alzheimer's disease. Biomed Res Int. 2014; 2014:927804.

13. Dunn NR, Pearce GL, Shakir SA. Adverse effects associated with the use of donepezil in general practice in England. J Psychopharmacol. 2000; 14:406–8.

14. Jackson S, Ham RJ, Wilkinson D. The safety and tolerability of donepezil in patients with Alzheimer's disease. Br J Clin Pharmacol. 2004; 58(Suppl 1):1–8.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download