This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Non-tunneled catheters (NTCs) are used for hemodialysis (HD) in many centers in which fluoroscopy is not easily accessed despite high complication rates and conditions requiring long-term HD. Therefore, here we aimed to evaluate the superiority of catheter-related outcomes after the application of tunneled cuffed catheter (TCC) without fluoroscopy versus unconditioned NTC insertion.
Methods
We divided the participants into two phases: those receiving NTCs between March 2010 and February 2011 (phase I), and those receiving TCCs or NTCs between March 2011 and February 2012 (phase II). Catheter survival, nurse satisfaction, and reasons for catheter removal were analyzed.
Results
Two hundred and sixty patients in phase I and 300 patients in phase II were enrolled in this study. The success rate of TCC insertion was 99.2%. The catheter survival rate in phase I was 65.5% at 1 month, while that in phase II was 74.9% at 1 month (p=0.023). We compared catheter survival between TCCs and NTCs for all periods regardless of phase. The TCC survival rate was higher than the NTC survival rate (p<0.001). Catheter-associated problems led to catheter removal in 97 patients (26.6%) in phase I and 68 patients (18.5%) in phase II (p=0.009). Among 14 HD nurses, all reported being satisfied with manipulation during pre-/post-HD, manupulation during HD, and overall. Eleven HD nurses (78.6%) reported being satisfied with the workload.
Conclusion
Compared with unconditional NTC insertion for HD, TCC insertion without fluoroscopy improved the overall catheter survival and nurse satisfaction rates.
Go to :

Keywords: Central venous catheter, Fluoroscopy, Hemodialysis, Renal dialysis
Introduction
The incidence and prevalence of end-stage renal disease (ESRD) continue to increase worldwide [
1-
3]. Hemodialysis (HD) is an established renal replacement therapy for patients with ESRD, and the adequate dialysis requires a vascular access, which is achieved via an arterio-venous fistula or graft for chronic HD. However, Yoon et al. [
4] showed that in 83.6% of patients initiating HD a central venous catheter is used as the first way of access in Korea.
HD catheters are divided into two groups according to the presence of cuff, that is, into temporary non-tunneled catheters (NTCs) and tunneled cuffed catheters (TCCs) [
5,
6]. NTCs are usually inserted into a femoral vein (FV) or an internal jugular vein (IJV), whereas TCCs are usually inserted into an IJV. TCCs are superior to NTCs in terms of infection rates, patient discomfort, and inadvertent removal [
7]. In addition, the Kidney Disease Outcomes Quality Initiative (KDOQI) guideline recommends that an NTC should only be used in hospitalized patients and for less than 1 week [
7].
Proper tip placement in TCCs is important to proper function. Fluoroscopy guidance for tip visualization would be considered mandatory for TCC insertion. However, NTCs may be inserted for HD in many centers in which fluoroscopy is not easily accessed despite high rates of complications and conditions requiring long-term HD. Therefore, we aimed to evaluate the superiority of catheter-related outcomes after the application of TCC without fluoroscopy versus unconditioned NTC insertion.
Go to :

Materials and methods
1. Ethics statement
This study was approved by the Institutional Review Board of Yeungnam University Hospital (IRB No: YUH-12-0401-052). The board waived the need for informed consent.
2. Study participants
We reviewed the medical records of Yeungnam University Medical Center in Korea to identify all adults (>18 years) who underwent HD catheter insertion between March 2010 and February 2012. In March 2011, our center employed a nephrologist with 2 years of experience with TCC insertion. NTCs were inserted between March 2010 and February 2011, while TCCs or NTCs were inserted between March 2011 and February 2012. HD catheter type was chosen by the nephrologist. Therefore, our study was divided into two phases: phase I (March 2010 to February 2011) and phase II (March 2011 to February 2012). Phases I and II were divided according to the application of TCC without fluoroscopy. In phase I, all HD catheters were inserted by NTC. In phase II, the patients requiring HD within 1 week were inserted with NTC and patients requiring HD >1 week were inserted with TCC without fluoroscopy. At phase I and II, NTC can be used for longer duration than 1–2 week according to clinical progress. Comparison between NTC and TCC using merged data may be associated with selection bias. Therefore, we have divided the study into two phases. NTCs and TCCs were used in the same manner during the two phases. We also compared the outcomes of NTC versus TCC without fluoroscopy regardless of phase.
3. Catheter insertion methods
TCCs were inserted as previously described [
8]. All were inserted routinely in a HD unit suite at our institution by two nurses and a nephrologist with 2 years of experience. Informed consent was obtained from all patients before the procedure. Briefly, the right (or left) neck and anterior chest wall were prepped with Betadine, and electrocardiography monitoring was utilized. Under ultrasonographic guidance (SA-8000; Medison, Seoul, Korea), the targeted IJV was punctured with a 21-gauge needle; after the confirmation of good venous return, a 0.018-inch guide wire was inserted (Mini Access Kit; Merit Medical Systems Inc., South Jordan, UT, USA), and a 4F sheath was placed. A pre-packaged Seldinger-type double lumen catheter set (14.5 Fr; Covidien, Mansfield, MA, USA or 14.5 Fr; Medcomp, Harleysville, PA, USA) was used that consisted of a double lumen catheter, two vessel dilators (11 and 13 Fr), a J-tip guide wire, a 15 Fr peel-away sheath, and a tunneler. A J-tip guide wire was advanced under electrocardiogram monitoring. Two skin incisions were made (access site, exit site). A tunnel was created and the catheter was then placed into the tunnel. Serial tract dilation was then performed to accommodate the peel-away sheath. The catheter was inserted using the peel-away sheath placement technique, and catheter function was checked by saline flushes with a 5-mL B.D syringe. Finally, the catheter tip was confirmed by chest X-ray as located within the heart shadow or heading for the right atrium or ventricle. If the catheter tip did not deviate widely from the heart shadow and HD catheter’s function was intact, the catheter was not repositioned. Techniques for NTC were same until it is insertion of a 4F sheath into relevant IJV or FV. A 0.038-inch guide wire was inserted and vessel dilator was inserted over the guidewire. Finally, HD catheter was inserted through the IJV (11 Fr; Medcomp) or FV (11.5 Fr; Medcomp).
4. Outcome measurements
The clinical data collected at the HD catheter insertion included age, disease underlying ESRD, sex, dialysis data, laboratory data, and HD catheter position/type, that is, NTC by the FV, NTC by IJV, or TCC by IJV. The data collected during follow-up included reasons for catheter removal classified as malfunction, infection, bleeding, neck edema, withdrawal from dialysis, death, follow-up loss, use of another access method (conversion from catheter to arterio-venous fistula, graft, or peritoneal dialysis), self-removal, and kidney transplantation. Malfunction, infection, bleeding, and neck edema were classified as catheter-associated problems. Catheter malfunction was defined as a negative pressure less than −250 mmHg at a blood flow rate<300 mL/min or frequent pressure alarm during the HD session for adequate dialysis [
7]. A catheter infection was diagnosed if pus, redness, induration, or tenderness was noted within 2 cm around the catheter exit site or along the catheter tunnel. A catheter infection also was diagnosed if catheter-related bacteremia was suspected. Bleeding was diagnosed if the exit or puncture site was oozing and treatment beyond a dressing change was needed. Neck edema was diagnosed if neck edema related to the catheter was suspected after the exclusion of other causes. In cases of catheter malfunction or infectious complications, the catheter was removed when the problems were not resolved by catheter manipulation or antibiotics. Technical success was defined as no procedure-associated complications with the catheter from venipuncture to HD initiation. Catheter survival was defined as the time that elapsed between catheter insertion and removal. Causes of catheter removal, excluding catheter-associated problems, were analyzed as censored data. Dialysis and laboratory data were reviewed at the time of HD initiation, and dialysis was specified as HD or continuous renal replacement therapy. Laboratory data included hemoglobin (g/dL), platelet count (K/mm
3), calcium (mg/dL), phosphorus (mg/dL), aspartate aminotransferase (AST; IU/L), alanine aminotransferase (ALT; IU/L), blood urea nitrogen (BUN; mg/dL), creatinine (mg/dL), sodium (mEq/L), and potassium (mEq/L).
For all 14 HD nurses who participated in both phases, satisfaction with TCC was assessed by four questions about manipulation during pre-/post-HD, manipulation during HD, workload, and total satisfaction. All 14 nurses responded very satisfied, satisfied, no change, dissatisfied, or very dissatisfied.
5. Statistical analysis
Continuous values are reported as mean±standard deviation and were compared using the t-test. Categorical variables are reported as count and percentage, and were compared using Pearson chi-square test or Fisher exact test. Kaplan-Meier analyses were used to identify the intergroup differences in survival. The p-values used for survival curve comparisons were calculated using the log-rank test. We performed Cox regression analyses of hazard ratio (95% confidence interval) for survival. Values of p<0.05 were considered statistically significant.
Go to :

Results
Two hundred and sixty patients in phase I and 300 patients in phase II were enrolled in this study (
Table 1). Sex and age did not differ significantly between groups. There were no significant intergroup differences in dialysis modality, hemoglobin, platelet count, calcium, phosphorus, AST, ALT, BUN, creatinine, or potassium level. Serum sodium levels were higher in phase II than in phase I. A total of 364 HD catheters were inserted in phase I versus 367 in phase II. Of them, 120 TCCs were inserted during phase II (32.7%). The TCC insertion success rate was 99.2%. One complication occurred during TCC insertion due to J-tip guidewire entrapment within the heart. This case was described in a previous paper [
9].
Table 1.
|
Variable |
Phase I |
Phase II |
p-valuea)
|
|
No. of patients |
260 |
300 |
- |
|
Underlying disease of ESRD (DM) |
123 (47.3) |
144 (48.0) |
0.870 |
|
No. of catheterizations |
364 |
367 |
- |
|
Age (yr) |
64.0±15.5 |
63.8±14.3 |
0.837 |
|
Male sex |
143 (55.0) |
177 (59.0) |
0.340 |
|
Hemoglobin (g/dL) |
9.6±1.8 |
9.8±1.9 |
0.216 |
|
Platelet (K/mm3) |
206±131 |
198±126 |
0.473 |
|
Calcium (mg/dL) |
7.7±1.2 |
7.5±1.0 |
0.297 |
|
Phosphorus (mg/dL) |
5.1±2.3 |
5.3±1.9 |
0.453 |
|
Aspartate aminotransferase (IU/L) |
215±817 |
200±496 |
0.829 |
|
Alanine aminotransferase (IU/L) |
102±317 |
103±252 |
0.987 |
|
Blood urea nitrogen (mg/dL) |
69.7±39.0 |
65.0±39.6 |
0.186 |
|
Creatinine (mg/dL) |
6.0±3.8 |
5.8±3.8 |
0.589 |
|
Sodium (mEq/L) |
135.2±6.4 |
136.9±6.7 |
0.002 |
|
Potassium (mEq/L) |
4.4±1.0 |
4.2±0.9 |
0.206 |
|
Continuous renal replacement therapy |
41 (15.8) |
46 (15.3) |
0.887 |
|
Type of hemodialysis catheter |
|
|
<0.001 |
|
Non-TCC (FV) |
176 (48.4) |
171 (46.6) |
|
|
Non-TCC (IJV) |
188 (51.6) |
76 (20.7) |
|
|
TCC (IJV) |
0 |
120 (32.7) |
|

The catheter survival rate during phase I was 65.5% at 1 month and 50.7% at 5 months, while that during phase II was 74.9% at 1 month and 63.7% at 5 months (
Fig. 1). Thus, the catheter survival rate during phase II was significantly higher than that during phase I (
p=0.023). Furthermore, TCC survival was significantly greater than NTC survival (
p<0.001).
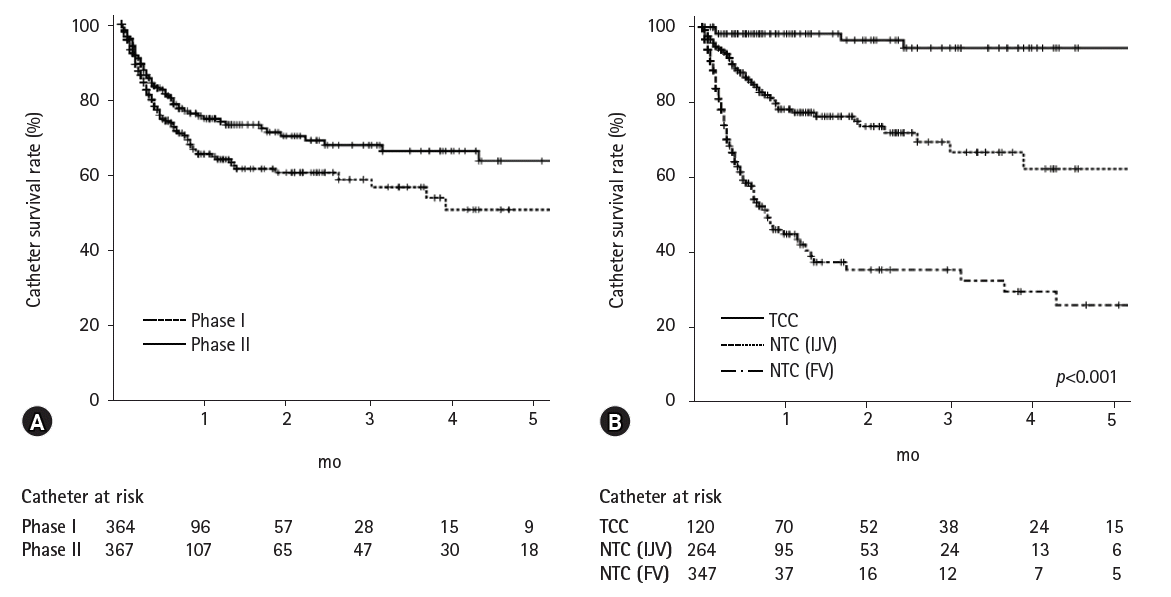 | Fig. 1.Catheter survival rate. (A) Plot by application of TCC (phase I, 65.5% at 1 month and 50.7% at 5 months; phase II, 74.9% at 1 month and 63.7% at 5 months). (B) Plot according to HD catheter type (NTC [FV], 44.7% at 1 month and 25.6% at 5 months; NTC [IJV], 77.6% at 1 month and 61.7% at 5 months; TCC, 98.2% at 1 month and 94.4% at 5 months). TCC, tunneled cuffed catheter; HD, hemodialysis; NTC, non-tunneled catheter; FV, femoral vein; IJV, internal jugular vein. 
|
Change in access was the most common cause of catheter removal in both phases (24.5% in phase I, 24.8% in phase II). However, the catheter removal rate for a catheter-associated problem was greater in phase I (26.6% in phase I, 18.5% in phase II;
p=0.009). The intervals from insertion to event in phases I and II were 33.4±74.6 and 34.9±58.4 days, respectively (
p=0.758). More specifically, in phase I, the removal rate for a catheter-associated problem was 33.0% for NTC by FV and 20.7% for NTC by IJV (
p=0.008). In phase II, the corresponding rates were 31.0% for NTC by FV, 14.5% for NTC by IJV, and 3.3% for TCC (
p<0.001). Catheters were removed due to patient death in phases I and II in 62 patients (17.0%) and 80 (21.8%), respectively (
p=0.103). Catheters were removed due to non-catheter-associated problems and catheter-associated problems in 267 patients (73.4%) and 97 patients (26.6%) in phase I and in 299 (81.5%) and 68 (18.5%) in phase II, respectively (
p=0.009). Catheter removal rates by catheter-associated problems were lower in phase II than in phase I.
Fig. 2 shows the specific causes of catheter removal.
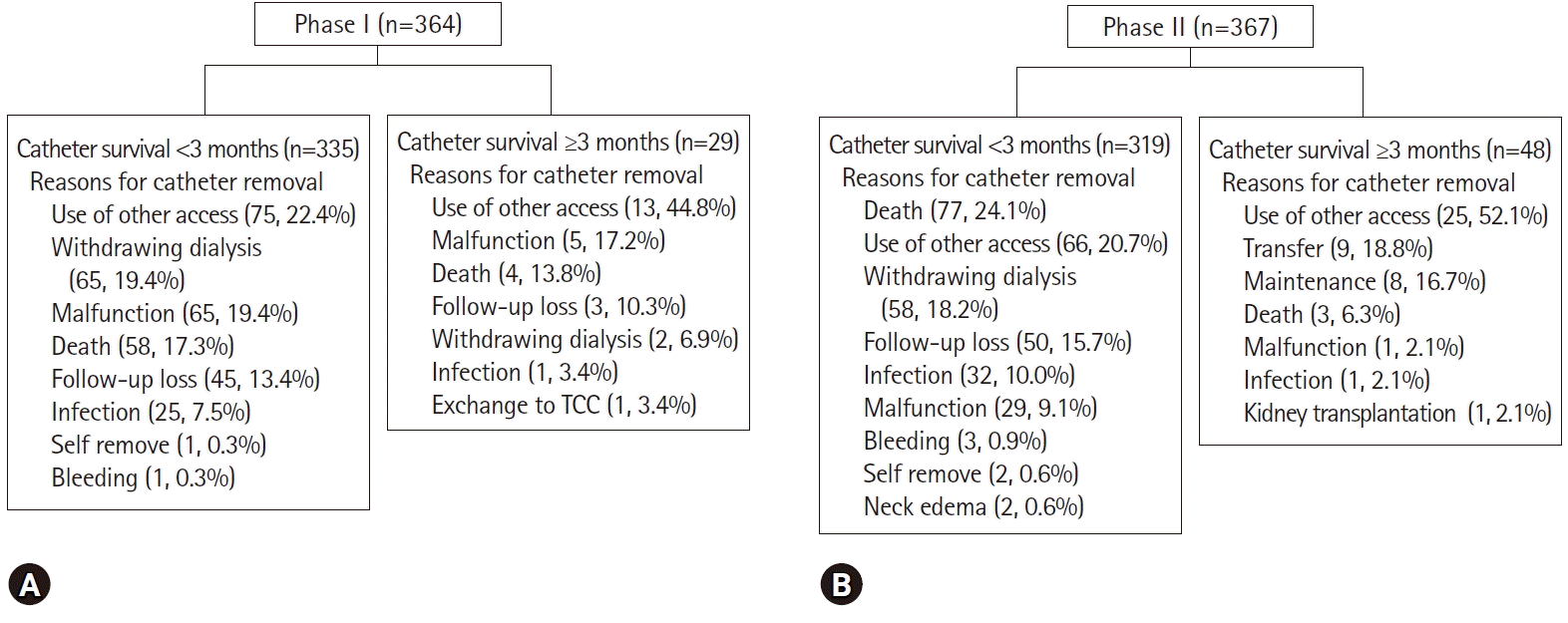 | Fig. 2.Study population and reasons of catheter removal by study period and catheter survival at 3 months after catheter insertion in (A) phase I, (B) phase II. TCC, tunneled cuffed catheter. 
|
We also analyzed the outcomes according to catheter type regardless of phase (
Fig. 3). The catheter survival rate was highest for TCC, while that for NTC (IJV) was higher than that for NTC (FV). There was no significant difference in catheter survival of the same catheter type between phases I and II. The number of catheters removed due to catheter-associated problems were 4 (3.3%), 39 (20.7%), 11 (14.5%), 58 (33.0%), and 53 (31.0%) for TCC, NTC (IJV) in phase I, NTC (IJV) in phase II, NTC (FV) in phase I, and NTC (FV) in phase II, respectively (
p<0.001). The proportion of removals due to catheter-associated problems for each catheter decreased after the application of TCC without fluoroscopy.
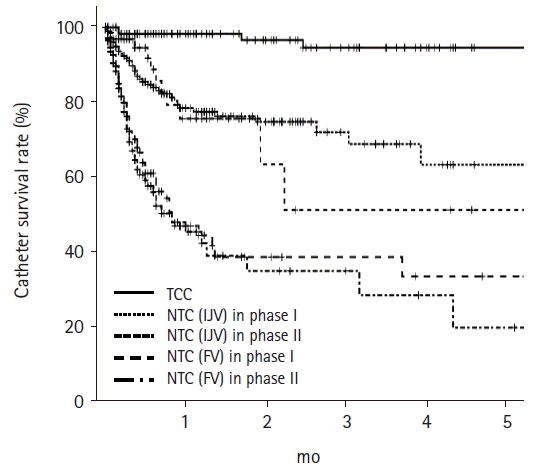 | Fig. 3.Catheter survival rates at 5 months for TCC, NTC (IJV) in phase I, NTC (IJV) in phase II, NTC (FV) in phase I, and NTC (FV) in phase II were 94.4%, 62.4%, 50.0%, 31.8%, and 17.8%, respectively. p<0.001 for TCC vs. the other catheters, NTC (IJV) in phase I or II vs. NTC (FV) in phase I or II, p=0.975 between NTC (IJV) in two phases, and p=0.494 between NTC (FV) in phases I and II. TCC, tunneled cuffed catheter; NTC, non-tunneled catheter; IJV, internal jugular vein; FV, femoral vein. 
|
Cox regression analysis, adjusted for diabetes, serum sodium, and old age (>65 years), showed a hazard ratio for catheter survival in phase II of 0.55 (95% confidence interval, 0.37–0.79;
p=0.002) (
Table 2). We performed subgroup analyses of age, sex, and diabetes. For elderly, male, or non-diabetic patients, there was a significantly lower risk of catheter removal due to catheter-associated problems in phase II than in phase I (
Table 3). We also performed Cox regression analyses for subgroup (
Table 4). The multivariate analysis was adjusted for diabetes mellitus, sex, serum sodium level, and phase for the age subgroup; for age, sex, serum sodium level, and phase for the diabetes mellitus subgroup; and for age, diabetes mellitus, serum sodium level, and phase for the sex subgroup. Elderly, diabetics, or male patients showed significantly greater overall catheter survival in phase II than in phase I. The other results showed similar trends, but statistical significance was not observed.
Table 2.
Cox regression analyses by variables
|
Variable |
Unadjusted |
Adjusted |
|
HR (95% CI) |
p-value |
HR (95% CI) |
p-value |
|
Age (ref: ≤65 yr) |
1.30 (0.96–1.77) |
0.096 |
1.03 (0.71–1.47) |
0.895 |
|
Comorbidity (ref: non-DM) |
1.31 (0.96–1.79) |
0.085 |
1.28 (0.88–1.85) |
0.199 |
|
Serum sodium (increase 1 unit) |
1.00 (0.97–1.03) |
0.872 |
1.00 (0.97–1.03) |
0.979 |
|
Phase II (ref: phase I) |
0.70 (0.51–0.96) |
0.024 |
0.55 (0.37–0.79) |
0.002 |

Table 3.
Removal due to catheter-associated problems according to subgroup of age, sex, or diabetes mellitus
|
Variable |
Phase I (n=97) |
Phase II (n=68) |
p-valuea)
|
|
Age<65 yr |
41 (17.6) |
30 (17.6) |
0.120 |
|
Age≥65 yr |
56 (28.6) |
38 (19.3) |
0.033 |
|
Male sex |
56 (27.9) |
37 (17.1) |
0.008 |
|
Female sex |
41 (25.2) |
31 (20.7) |
0.346 |
|
Non-diabetes mellitus |
57 (28.8) |
38 (20.1) |
0.047 |
|
Diabetes mellitus |
40 (24.1) |
30 (16.9) |
0.095 |

Table 4.
Cox regression analyses of subgroup of age, sex, or diabetes mellitus
|
Variable |
Unadjusted |
Adjusted |
|
HR (95% CI) |
p-value |
HR (95% CI) |
p-value |
|
Age<65 yr |
|
|
|
|
|
Sex (ref: male) |
1.26 (0.77–1.95) |
0.392 |
1.23 (0.72–2.11) |
0.443 |
|
Comorbidity (ref: non-DM) |
1.81 (1.13–2.91) |
0.014 |
1.73 (1.02–2.93) |
0.043 |
|
Serum sodium (increase 1 unit) |
1.00 (0.96–1.04) |
0.911 |
1.00 (0.96–1.05) |
0.927 |
|
Phase II (ref: phase I) |
0.71 (0.44–1.13) |
0.144 |
0.65 (0.37–1.12) |
0.122 |
|
Age≥65 yr |
|
|
|
|
|
Sex (ref: male) |
0.84 (0.55–1.27) |
0.402 |
1.00 (0.59–1.68) |
0.998 |
|
Comorbidity (ref: non-DM) |
1.01 (0.67–1.53) |
0.947 |
0.96 (0.57–1.62) |
0.885 |
|
Serum sodium (increase 1 unit) |
1.00 (0.96–1.03) |
0.863 |
1.00 (0.97–1.04) |
0.922 |
|
Phase II (ref: phase I) |
0.70 (0.46–1.07) |
0.097 |
0.49 (0.29–0.83) |
0.008 |
|
Male sex |
|
|
|
|
|
Age (ref: ≤65 yr) |
1.53 (1.01–2.31) |
0.044 |
1.02 (0.63–1.66) |
0.938 |
|
Comorbidity (ref: non-DM) |
1.54 (1.01–2.34) |
0.044 |
1.43 (0.86–2.37) |
0.167 |
|
Serum sodium (increase 1 unit) |
0.99 (0.95–1.02) |
0.460 |
0.99 (0.95–1.02) |
0.525 |
|
Phase II (ref: phase I) |
0.58 (0.38–0.88) |
0.011 |
0.47 (0.28–0.78) |
0.003 |
|
Female sex |
|
|
|
|
|
Age (ref: ≤65 yr) |
1.05 (0.66–1.68) |
0.830 |
0.84 (0.47–1.49) |
0.551 |
|
Comorbidity (ref: non-DM) |
1.06 (0.67–1.69) |
0.799 |
1.12 (0.64–1.94) |
0.701 |
|
Serum sodium (increase 1 unit) |
1.01 (0.97–1.06) |
0.567 |
1.02 (0.97–1.06) |
0.476 |
|
Phase II (ref: phase I) |
0.88 (0.55–1.41) |
0.594 |
0.72 (0.40–1.30) |
0.273 |
|
Non-DM |
|
|
|
|
|
Sex (ref: male) |
0.85 (0.56–1.29) |
0.452 |
0.95 (0.59–1.56) |
0.850 |
|
Age (ref: ≤65 yr) |
0.98 (0.65–1.47) |
0.916 |
0.76 (0.48–1.23) |
0.268 |
|
Serum sodium (increase 1 unit) |
0.98 (0.95–1.02) |
0.346 |
0.99 (0.95–1.02) |
0.508 |
|
Phase II (ref: phase I) |
0.83 (0.55–1.25) |
0.369 |
0.68 (0.41–1.11) |
0.125 |
|
DM |
|
|
|
|
|
Sex (ref: male) |
1.26 (0.79–2.02) |
0.332 |
1.24 (0.71–2.19) |
0.450 |
|
Age (ref: ≤65 yr) |
1.79 (1.11–2.89) |
0.017 |
1.44 (0.82–2.52) |
0.207 |
|
Serum sodium (increase 1 unit) |
1.02 (0.98–1.07) |
0.406 |
1.03 (0.98–1.08) |
0.241 |
|
Phase II (ref: phase I) |
0.60 (0.37–0.96) |
0.033 |
0.42 (0.23–0.75) |
0.003 |

Among the 14 HD nurses, all reported being satisfied with the manipulation during pre-/post-HD, manipulation during HD, and overall. Eleven HD nurses (78.6%) reported being satisfied with the workload.
Go to :

Discussion
Our study showed that the rate of catheter removal due to catheter-associated problems was lower in phase II than in phase I. The both univariate and multivariate analyses showed that the overall catheter survival rate was higher in phase II than in phase I. For elderly, male, or non-diabetic patients, there was a significant lower risk of catheter removal due to catheter-associated problems in phase II than in phase I. For younger, female, or diabetic patients, similar trends were shown, but no statistical significance was observed. For elderly, male, or diabetic patients, catheter survival rates were higher in phase II than in phase I. For younger, female, or non-diabetic patients, catheter survival was favorable in phase II, but statistical significance was not observed. Nurse satisfaction rates were higher in phase II than in phase I.
Fluoroscopy is not always available at HD centers because it is expensive and requires materials to protect staff from radiation exposure. TCCs have been inserted in many centers by interventional radiologists familiar with fluoroscopy. TCCs are now being inserted by interventional nephrologists who can perform the procedures necessary for access management. We wanted to evaluate the superiority of TCC without fluoroscopy versus unconditioned NTC, even when fluoroscopy was not available. Catheter outcomes of TCC are undoubtedly better than those of NTC. Oliver reviewed HD catheter infections and showed a higher risk in NTC versus TCC [
10]. Wang et al. [
11] investigated 865 dialysis patients and demonstrated a 3.49 odds ratio for catheter dysfunction in NTC versus TCC on multivariate analysis. Mendu et al. [
12] showed that TCC had improved dialysis delivery and lower mechanical complication rates than NTC. In our study, 4 (3.3%) and 161 (26.4%) TCC and NTC catheters were removed due to catheter-associated problems, respectively (
p<0.001). However, NTC may be used for HD in many centers in which fluoroscopy is not easily accessed despite high complication rates of NTC and conditions requiring long-term HD.
In our hospital, all patients requiring HD were inserted using NTC regardless of the needs for long-term catheterization during phase I. In phase II, although fluoroscopy was not available, we try to use TCCs for patients requiring relatively long-term HD. The success rate of TCC insertion without fluoroscopy was high and only one immediate catheter insertion-related complication was observed. In addition, TCC insertion without fluoroscopy significantly improved the rates of overall catheter survival and catheter removal due to catheter-associated problems. Fluoroscopy is usually used to identify catheter tip position and prevent arterial catheter insertion or puncture. A previous study compared TCC insertion with or without fluoroscopy and found that TCC insertion without fluoroscopy was associated with reduced immediate success rate [
13]. However, our study showed favorable results for TCC insertion with versus without fluoroscopy and that the application of TCC without fluoroscopy improved overall catheter survival, catheter-associated complication, and nurse satisfaction rates versus unconditioned NTC insertion.
The KDOQI guideline recommends that TCC be used in patients requiring dialysis for longer than a week and that TCCs should be inserted in centers in which ultrasonography and fluoroscopy are available [
7]. However, most patients requiring emergent HD cannot be withdrawn from HD within 1 week therefore, most HD catheters used for emergent HD are a TCCs. A study using a NTC showed that the mean dialysis session was 11.3±6.8 and catheters were left in place for a mean 19.5±15.3 days [
14]. Beathard et al. [
15] reported 1,765 cases of TCC placement and 2,262 of TCC exchange. The success rates were 98.24% and 98.36%, respectively, and the complication rates were 1.42% and 1.41%, respectively. Motta Elias et al. [
16] reported their single-center experiences with 130 catheter exchanges from NTC to TCC without fluoroscopy. In their study, the catheter survival rate was 68.0% at 120 days and the catheter removal rate for a catheter-associated problem was 37%. However, although this previous study was prospective, it involved a single arm and only catheter exchange from NTC to TCC. Furthermore, catheter survival was lower in the in this previous study than in the present study. In the present study, the study period were divided into two phases and the TCC survival rate at 5 months was 94.4%.
This study is limited by its retrospective and single-center nature. Furthermore, we could not evaluate complications during HD regardless of catheter removal. In addition, all TCCs were inserted by a nephrologist, as practitioner skill can obviously affect complication rates. Therefore, we suggest the need for a larger prospective multi-center study.
In summary, our study showed that TCC insertion without fluoroscopy can be performed with high success rates. Compared with unconditional NTC insertion for HD, the application of TCC insertion without fluoroscopy improved the overall catheter survival and nurse satisfaction rates. Although fluoroscopy was not accessible, TCC insertion without fluoroscopy would help improve catheter-related outcomes.
Go to :







 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download