Discussion
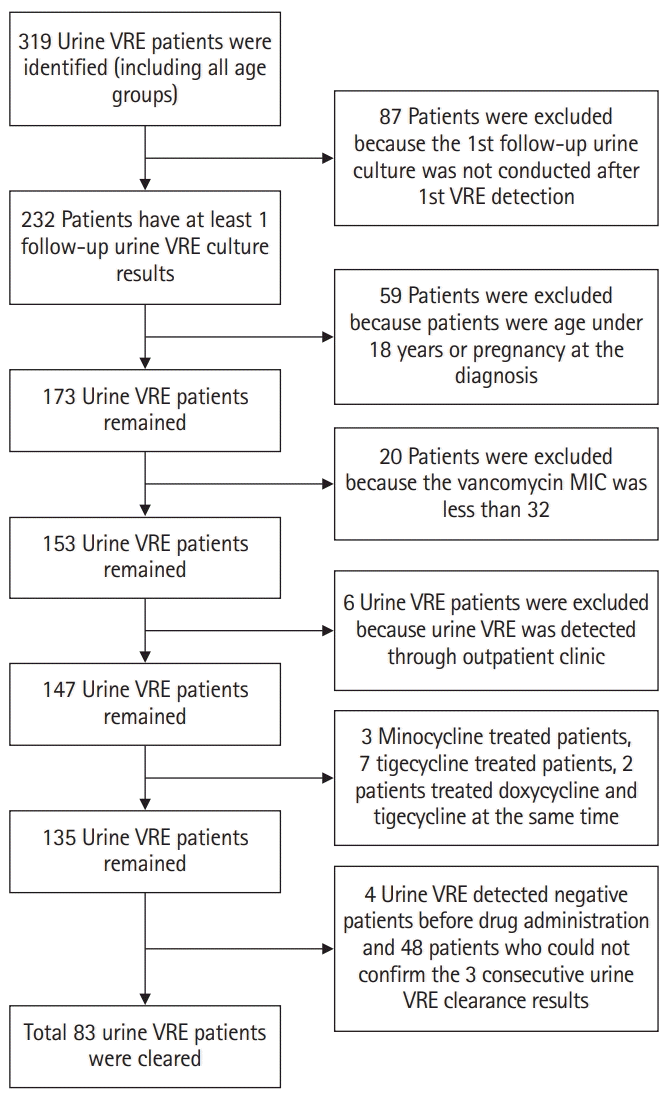
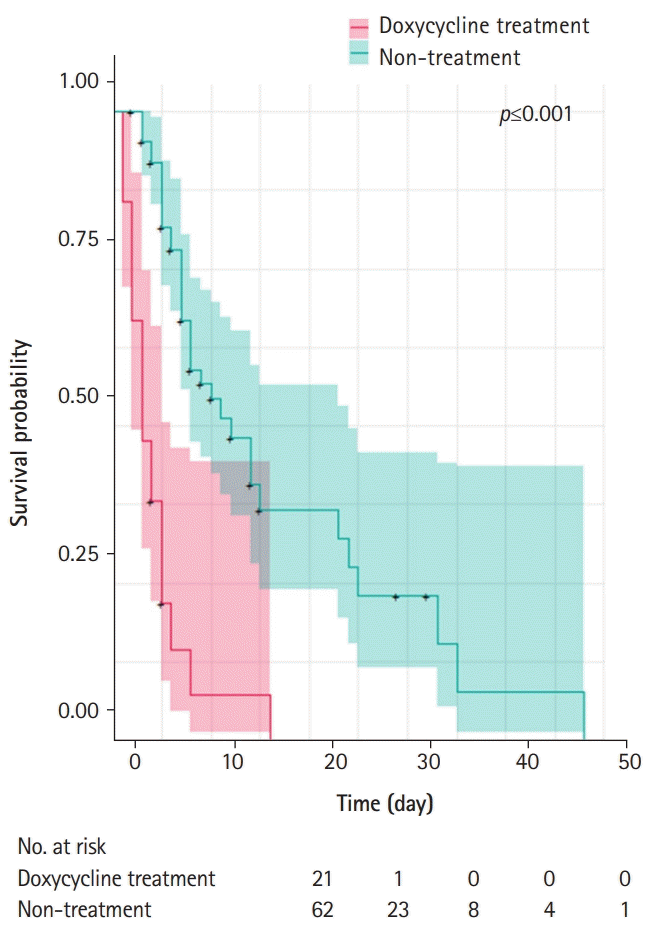
This was the first study to show that oral doxycycline treatment affected urine VRE colonization. In our study, oral doxycycline treatment increased the overall eradication rate of urine VRE and enhanced the eradication process. In particular, taking into account the possibility of natural VRE decolonization, our results showed that doxycycline increased the eradication rate regardless of the other antibiotics administered during the same period.
Preservation of antibiotic effectiveness and prevention of adverse effects associated with unnecessary antibiotic use are of utmost importance in all healthcare systems [
8]. However, with the emergence of multidrug-resistant pathogens, there are difficulties in the selection of suitable antibiotics.
Eradication of VRE colonization remains a great challenge for infection-control professionals. To reduce or eradicate VRE colonization, various approaches, such as pharmacological treatments, use of bacteriophages, and fecal transplantation, have been previously assessed. However, there are several limitations to the clinical commercialization of these treatment options, and additional studies are needed.
Doxycycline is a bacteriostatic agent that reversibly binds to ribosomal units and inhibits bacterial protein synthesis. Approximately 90%–100% of this drug is absorbed after oral administration [
9]. Doxycycline concentration is the highest in the liver, kidney, and digestive tract, which are excretory organs. Doxycycline is eliminated unchanged via both the renal and biliary routes. Approximately 35%–60% of this drug is excreted via urine and the remainder via feces [
10]. Peak levels of doxycycline are reached at approximately 2–4 hours after administration. Its plasma half-life is 18–22 hours in adults with normal renal function, and 20–30 hours in patients with severe renal impairment [
11].
In the past years, decolonization of the gastrointestinal tract has been investigated as a method for the prevention of VRE infection in vulnerable patient groups. Various oral antimicrobial regimens have been evaluated for gastrointestinal VRE decolonization. Single agents and combinations of several antimicrobial agents have been used [
3]. In one prospective observational cohort study, patients were treated with bacitracin solution (75,000 units daily) and doxycycline capsules (100 mg daily) for 14 days. At the end of the 14-day treatment period, all 15 treated patients (100%) showed stool VRE clearance, with only eight of the untreated patients (33%) were VRE-free. However, there was no difference in the frequency of intermittent or persistent VRE colonization at 4 months between the two groups, suggesting that oral bacitracin and doxycycline treatments were not effective in reducing the carriage of VRE beyond the 2-week interval and did not exert a long-term effect on VRE colonization [
7]. In our study, 100 mg doxycycline was administered orally twice a day, which was double the dose administered in previous studies. In addition, the median time for urine VRE eradication was identified to be 8 days, which was shorter compared with that in former rectal swab VRE studies. Moreover, we could not identify the persistence or eradication of gastrointestinal VRE. Because our study was conducted retrospectively, a rectal swab culture was not routinely performed during the study period. However, our study presented the possibility of reducing the propagation of surrounding contaminations caused by urine VRE.
It is challenging to assess the clinical significance of VRE in routine cultures or to differentiate colonization from infection, especially when VRE is detected in urine as part of a polymicrobial infection, as it is a colonizer of the genitourinary tract and often results in asymptomatic bacteriuria [
12]. A previous retrospective study showed that most patients with VRE bacteriuria were classified as colonization and asymptomatic bacteriuria, and only 25% of patients with VRE bacteriuria required antibiotic therapy. Moreover, among urine VRE strains,
E. faecium was identified in the urine culture of 99 patients (68.8%), whereas
E. faecalis was identified in 45 patients (31.2%) [
13]. In our study,
E. faecium was identified in the urine culture of 81 patients (97.6%), whereas
E. faecalis was identified only in two patients (2.4%). Pyuria was identified in 17 patients (20.5%). The accuracy of this result might have been affected by previous use of other antibiotics. However, most of the patients did not have urinary symptoms, indicating that the risk of VRE urinary tract infection (UTI) is low, and most of the urine VRE-positive patients in hospitals can be considered positive for VRE colonization.
When VRE is detected in urine culture samples, if there are no signs of UTI; thus, waiting for natural decolonization or discontinuation of antibiotics within a short time can be considered. However, in certain clinical situations, such as when the cause of the infection is unclear, when persistent long-term antibiotic treatment is required, or when VRE is continuously identified in severely ill patients with immunocompromised diseases in a hospital setting, the need for antibiotic administration might arise. Environmental contamination can increase the risk of VRE acquisition [
14]. VRE can be transmitted from patient to patient any time; therefore, careful contact precautions must be taken. Our study showed that doxycycline treatment for urine VRE colonization was effective in shortening the urine VRE isolation period; however, considering the natural urine VRE decolonization period, decolonization treatment must be administered to carefully selected patients who need it.
A previous study showed that 4% (31/768) of all VRE-colonized patients developed VRE blood stream infection (BSI), and the independent risk factors for death included immunosuppression and VRE BSI [
15]. VRE infections tend to occur in more debilitated or seriously ill hospitalized patients. Mortality rate in patients with VRE BSI can reach up to 70% [
16-
18]. In our study, VRE BSI was detected in 0.012% of the patients (1/83). VRE BSI was not detected in the doxycycline treatment group, but was detected in one patient with myelodysplastic syndrome who did not receive doxycycline treatment. Furthermore, no deaths were observed in all patients regardless of doxycycline administration. However, previous studies have reported that patients with neutropenia, organ transplants, dialysis, or hematologic malignancy are at a high risk of experiencing prolonged bacteremia or death due to VRE infection [
19,
20]. Doxycycline treatment for urine VRE decolonization might have protective effects against severe bacteremia in certain high-risk patients, but further prospective studies are needed for verification. According to a recent study, stool VRE colonization appears to be an independent risk factor for
Clostridioides difficile infection recurrence [
21]. Therefore, decolonization of the gastrointestinal tract, the primary reservoir for VRE, might be useful in certain immunocompromised patients with hematologic diseases or those who have undergone organ transplantation. In our study, a rectal swab study was not performed. However, if urine VRE is detected in patients positive for rectal swab VRE, doxycycline treatment can be considered, as doxycycline is also excreted via feces.
As our results did not allow us to conclude whether doxycycline treatment prevented sepsis development, further prospective studies with a larger number of patients should be performed to validate this relationship and the pathophysiology between VRE eradication and doxycycline. We hypothesize that, by reducing urine VRE colonization, doxycycline treatment may reduce the number of deaths due to VRE bacteremia.
Cephalosporin antibiotics have become a major part of the antibiotic formulary of hospitals, and are prescribed for various infections, including UTI. Prior use of cephalosporin antibiotics is a major risk factor for enterococcal infections [
22,
23]. The most common infections caused by VRE are UTIs, bacteremia, and wound infections [
24]. Risk factors for VRE acquisition include colonization pressure, use of antimicrobials, old age, diabetes, installation of a urinary catheter, severe illness (especially end-stage renal diseases requiring dialysis), cancer, and previous transplants [
25]. In our study, once urine VRE was acquired, among the modifiable factors, Foley catheter change did not lower urine VRE persistence rate, whereas doxycycline administration decreased VRE colonization and persistency rate. Hence, for patients requiring continuous immunosuppressive therapy and continued use of restriction antibiotics, urine VRE can be considered as a therapeutic target. Current guidelines regarding asymptomatic bacteriuria or VRE-associated urinary colonization are observation without any pharmacological treatment. Specific management guidelines for urine VRE for various population groups have not been published. This study provided information that might help elucidate the pharmacological treatments needed for certain patient groups.
However, this study had several limitations. First, this was a single-center study with a relatively small study population. Second, as this was a retrospective study, we could not perform rectal swab cultures. When urine VRE was detected, stool samples were not collected at the same time. Hence, further studies are required for simultaneous stool and urine VRE screening. Third, the follow-up urine culture time was not consistent. We followed the revised guidelines of the Korea Centers for Disease Control and Prevention on VRE management [
26]. According to the guidelines, culture follow-up intervals range from 3 days to 1 week, but in our study, culture follow-up time showed an irregular tendency. If the culture follow-up interval is long, there may be a difference in VRE eradication rate due to the longer duration taken to obtain negative culture results. However, no significant difference in the average culture interval was observed between the doxycycline treatment and non-treatment groups. Additional prospective studies are needed to further validate our results.
The current VRE isolation criterion is the confirmation of three consecutive negative VRE surveillance cultures conducted at least once a week. To date, VRE decolonization studies are usually conducted at 1-week intervals. However, in a typical clinical setting where isolation rooms are limited, culture trials are often implemented at least twice a week on average after VRE confirmation. Here, we established a model that mimicked an actual clinical setting. Our study included severely ill patients who were subjected to continued antibiotic use, and our results showed that additional doxycycline treatment significantly increased urine VRE eradication rate.
In conclusion, doxycycline treatment for urine VRE colonization appeared to be useful in shortening the urine VRE isolation period, reducing the risk for further nosocomial spread of VRE, and lowering the need for prolonged isolation. Achieving a high eradication rate can shift the treatment paradigm and offer clinicians an alternative to the traditional anti-VRE agents for the management of urine VRE colonization. Moreover, identifying appropriate antibiotic therapies for urine VRE colonization might play an important role in improving antimicrobial stewardship.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download