1. Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002; 360:1267–74.

2. Regli L, Uske A, de Tribolet N. Endovascular coil placement compared with surgical clipping for the treatment of unruptured middle cerebral artery aneurysms: a consecutive series. J Neurosurg. 1999; 90:1025–30.

3. Regli L, Dehdashti AR, Uske A, de Tribolet N. Endovascular coiling compared with surgical clipping for the treatment of unruptured middle cerebral artery aneurysms: an update. Acta Neurochir Suppl. 2002; 82:41–6.

4. Raftopoulos C, Mathurin P, Boscherini D, Billa RF, van Boven M, Hantson P. Prospective analysis of aneurysm treatment in a series of 103 consecutive patients when endovascular embolization is considered the first option. J Neurosurg. 2000; 93:175–82.

5. Iijima A, Piotin M, Mounayer C, Spelle L, Weill A, Moret J. Endovascular treatment with coils of 149 middle cerebral artery berry aneurysms. Radiology. 2005; 237:611–9.

6. Doerfler A, Wanke I, Goericke SL, Wiedemayer H, Engelhorn T, Gizewski ER, et al. Endovascular treatment of middle cerebral artery aneurysms with electrolytically detachable coils. AJNR Am J Neuroradiol. 2006; 27:513–20.
7. Quadros RS, Gallas S, Noudel R, Rousseaux P, Pierot L. Endovascular treatment of middle cerebral artery aneurysms as first option: a single center experience of 92 aneurysms. AJNR Am J Neuroradiol. 2007; 28:1567–72.

8. Horowitz M, Gupta R, Gologorsky Y, Jovin T, Genevro J, Levy E, et al. Clinical and anatomic outcomes after endovascular coiling of middle cerebral artery aneurysms: report on 30 treated aneurysms and review of the literature. Surg Neurol. 2006; 66:167–71.

9. Kim J, Chang C, Jung Y. Feasibility and midterm outcomes of endovascular coil embolization of an unruptured middle cerebral artery aneurysm with an incorporated branch. World Neurosurg. 2018; 118:e745–52.

10. Kim BM, Park SI, Kim DJ, Kim DI, Suh SH, Kwon TH, et al. Endovascular coil embolization of aneurysms with a branch incorporated into the sac. AJNR Am J Neuroradiol. 2010; 31:145–51.

11. Lubicz B, Leclerc X, Gauvrit JY, Lejeune JP, Pruvo JP. HyperForm remodeling-balloon for endovascular treatment of wide-neck intracranial aneurysms. AJNR Am J Neuroradiol. 2004; 25:1381–3.
12. Lubicz B, Lefranc F, Levivier M, Dewitte O, Pirotte B, Brotchi J, et al. Endovascular treatment of intracranial aneurysms with a branch arising from the sac. AJNR Am J Neuroradiol. 2006; 27:142–7.
13. Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001; 32:1998–2004.

14. Ausman JI. The future of neurovascular surgery. Part I: intracranial aneurysms. Surg Neurol. 1997; 48:98–100.

15. Suzuki S, Tateshima S, Jahan R, Duckwiler GR, Murayama Y, Gonzalez NR, et al. Endovascular treatment of middle cerebral artery aneurysms with detachable coils: angiographic and clinical outcomes in 115 consecutive patients. Neurosurgery. 2009; 64:876–88.
16. Bracard S, Abdel-Kerim A, Thuillier L, Klein O, Anxionnat R, Finitsis S, et al. Endovascular coil occlusion of 152 middle cerebral artery aneurysms: initial and midterm angiographic and clinical results. J Neurosurg. 2010; 112:703–8.

17. Jayaraman MV, Do HM, Versnick EJ, Steinberg GK, Marks MP. Morphologic assessment of middle cerebral artery aneurysms for endovascular treatment. J Stroke Cerebrovasc Dis. 2007; 16:52–6.

18. Higashida RT, Lahue BJ, Torbey MT, Hopkins LN, Leip E, Hanley DF. Treatment of unruptured intracranial aneurysms: a nationwide assessment of effectiveness. AJNR Am J Neuroradiol. 2007; 28:146–51.
19. van Rooij WJ, Sluzewski M. Procedural morbidity and mortality of elective coil treatment of unruptured intracranial aneurysms. AJNR Am J Neuroradiol. 2006; 27:1678–80.
20. Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005; 366:809–17.

21. Choi JH, Park JE, Kim MJ, Kim BS, Shin YS. Aneurysmal neck clipping as the primary treatment option for both ruptured and unruptured middle cerebral artery aneurysms. J Korean Neurosurg Soc. 2016; 59:269–75.

22. Brinjikji W, Lanzino G, Cloft HJ, Rabinstein A, Kallmes DF. Endovascular treatment of middle cerebral artery aneurysms: a systematic review and single-center series. Neurosurgery. 2011; 68:397–402.
23. Kim SR, Vora N, Jovin TG, Gupta R, Thomas A, Kassam A, et al. Anatomic results and complications of stent-assisted coil embolization of intracranial aneurysms. Interv Neuroradiol. 2008; 14:267–84.

24. Nishido H, Piotin M, Bartolini B, Pistocchi S, Redjem H, Blanc R. Analysis of complications and recurrences of aneurysm coiling with special emphasis on the stent-assisted technique. AJNR Am J Neuroradiol. 2014; 35:339–44.

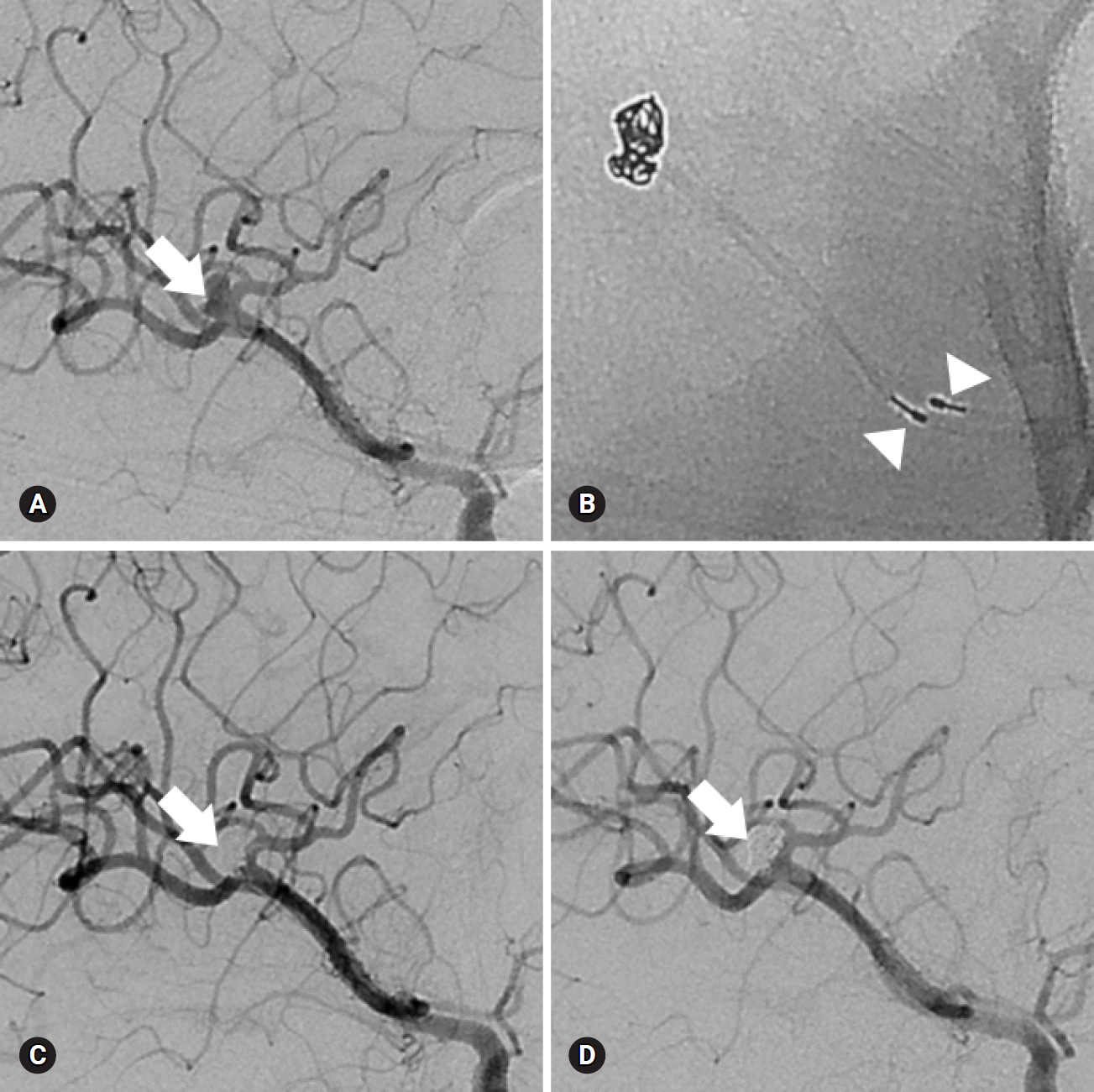
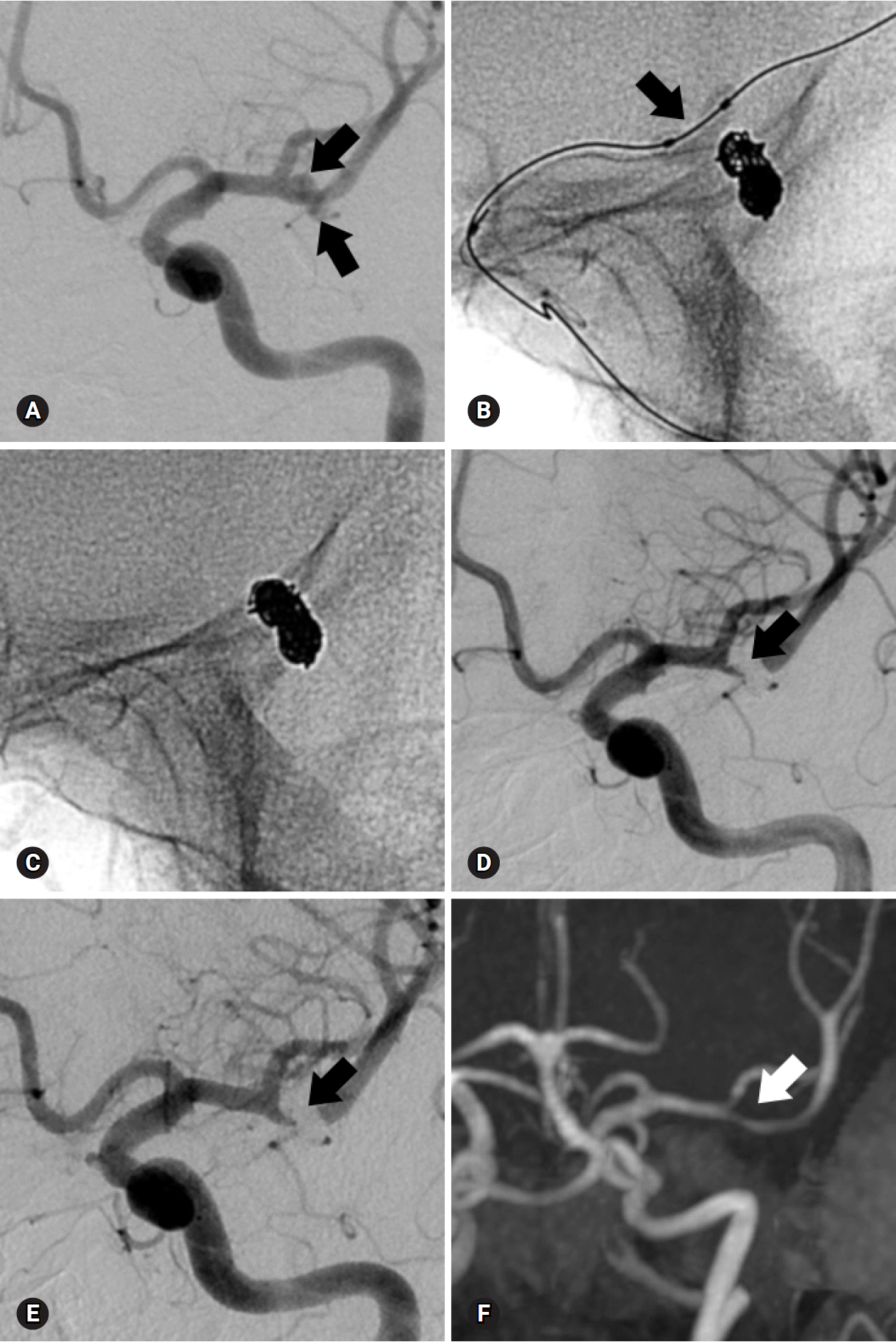
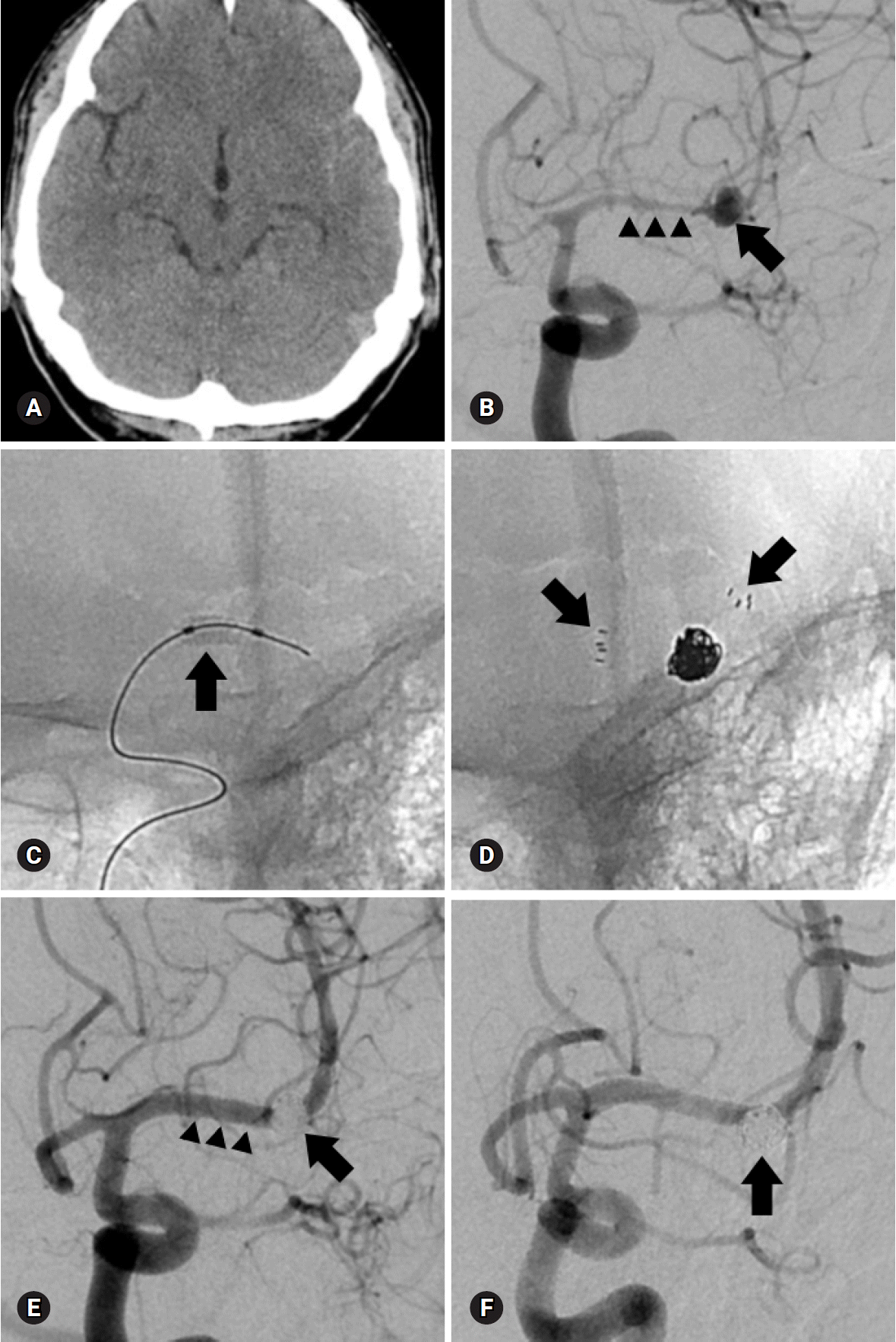




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download