Abstract
Idiopathic non-cirrhotic portal hypertension (INCPH) is a disease with an uncertain etiology consisting of non-cirrhotic portal hypertension and portal pressure increase in the absence of liver cirrhosis. In INCPH, patients exhibit normal liver functions and structures. The factors associated with INCPH include the following: Umbilical/portal pyremia, bacterial diseases, prothrombic states, chronic exposure to arsenic, vinyl chloride monomers, genetic disorders, and autoimmune diseases. Approximately 70% of patients present a history of major variceal bleeding, and treatment relies on the prevention of complications related to portal hypertension. Autoimmune disorders associated with INCPH are mainly systemic sclerosis, systemic lupus erythematosus and rheumatoid arthritis. To the best of our knowledge, a case of ankylosing spondylitis (AS) associated with INCPH has not been reported thus far. Therfore, we report our experience of a patient with AS accompanied by INCPH, who showed perisplenic varices with patent spleno-portal axis and hepatic veins along with no evidence of cirrhosis on liver biopsy, and provide a brief literature review.
Idiopathic non-cirrhotic portal hypertension (INCPH) is a rare condition characterized by increased portal pressure with patent portal and hepatic veins in the absence of cirrhosis. The underlying pathophysiologic mechanisms of INCPH still remain unclear. INCPH has been associated with various conditions, including immunologic disorders, recurrent infections, medications and toxins, genetic disorders, and prothrombic conditions [1]. Histological features involve a wide range of non-specific changes, including sinusoidal dilatation, phlebosclerosis, portal fibrosis, and nodular regenerative hyperplasia [2]. Moreover, INCPH is a diagnosis of exclusion, and the diagnosis is made when the following criteria are fulfilled: (1) evidence of portal hypertension, (2) doppler ultrasound showing patent portal and hepatic veins, (3) liver biopsy showing no cirrhosis, (4) exclusion of conditions that cause cirrhosis, such as chronic viral hepatitis, alcoholic liver disease, nonalcoholic steatohepatitis, obesity, or autoimmune hepatitis, and (5) exclusion of chronic vitamin A intake, exposure to copper sulphate, vinyl chloride monomer, or past angiography with thrombin sulphate [3]. Treatment is aimed to prevent potential complications of portal hypertension, and unfortunately, there is no available treatment to date that modifies the course of the disease or prevents complications.
We experienced a rare case of INCPH in a patient with ankylosing spondylitis (AS). INCPH is associated with immunological disorders, but it is mainly reported in patients with systemic sclerosis (SS), systemic lupus erythematosus (SLE), autoimmune hepatitis, and rheumatoid arthritis (RA) [1,4,5]. To date, INCPH in a patient with AS has not been reported. Therefore, we report INCPH in a patient with AS along with a brief literature review.
An 82-year-old female patient presented lower back pain that has been aggravated for the last 1 month. She was initially diagnosed with AS 16 years ago at a local clinic. She had been taking acetaminophen 325mg, tramadol 37.5mg, and aceclofenac 100mg, twice a day. She underwent both total hip replacement due to degenerative changes. She denied using alcohol and tobacco as well as taking any vitamins or other dietary supplements. She was not considered obese (body mass index 22.6 kg/m2). The modified Schobers test was positive (3.5 cm); the fingertip to ground distance was 7 cm; and chest expansion measured 3.5 cm, which is considered to be outside the normal range. There was no flexion deformity of the cervical spine. Additionally, there was no tenderness at the sacroiliac joint on physical examination, and she showed no signs of arthritis or enthesitis.
On laboratory findings, the erythrocyte sedimentation rate was 47mm/hr (range, 0-30mm/hr) and high-sensitivity C-reactive protein was 18.22mg/L (range, 0-0.5mg/L). Blood cell count was normal with platelet counts of 282,000/μL. Human leukocyte antigen-B27, antinuclear antibody, and antiphopholipid antibodies were negative. Furthermore, rheumatoid factor was 6.8 IU/mL (range, 0-15 IU/mL). Liver function tests and PT/INR were normal. HBsAg, antiHBs, and antiHCV were negative. There was no evidence of schistosomiasis on the serologic test.
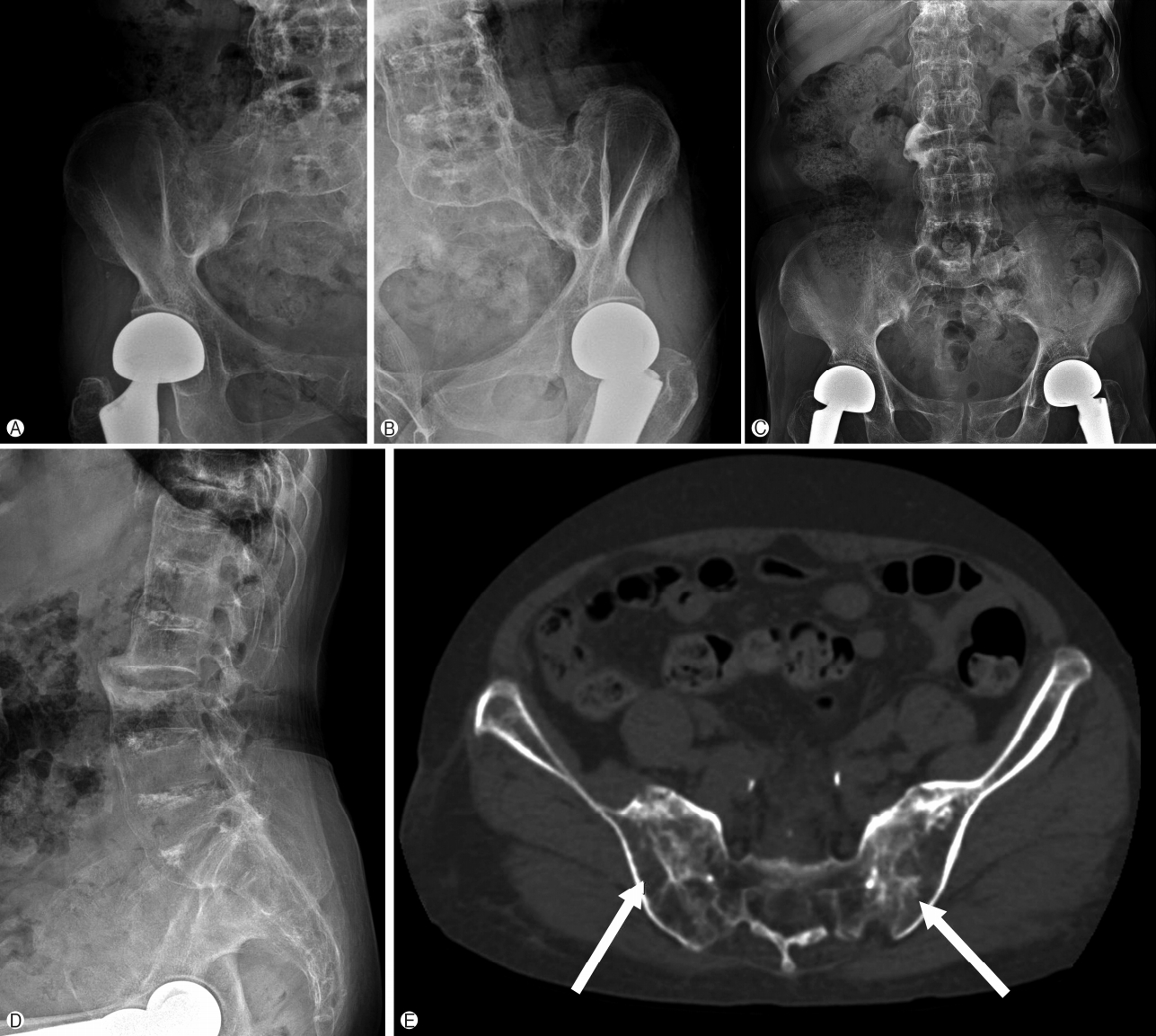
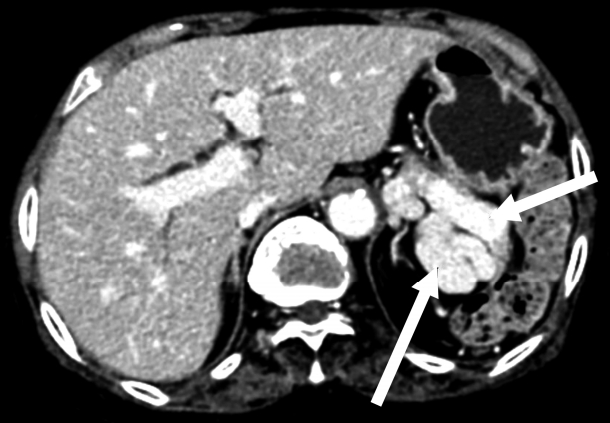
On radiological findings, the sacroiliac joint on lumbar X-ray and computed tomography (CT) showed bilateral sacroiliitis (grade 4) and bamboo spine consistent with AS (Fig. 1). Perisplenic varices and chronic obstructive nephropathy were observed on the abdominal CT(Fig. 2). Splenomegaly was not found. Doppler ultrasound showed patent portal and hepatic veins with no evidence of portal or splenic vein thrombosis.
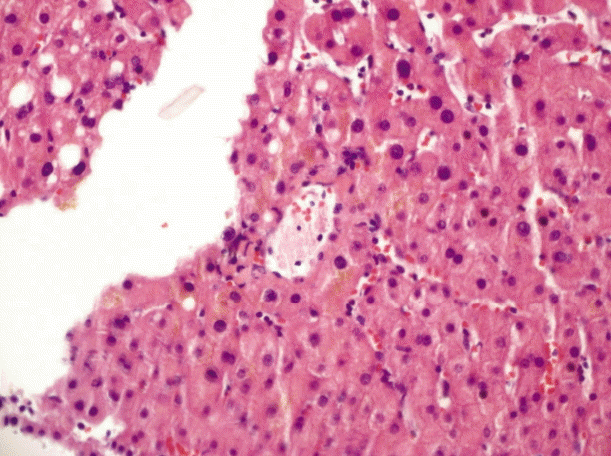
We discovered gastric ulcer on upper gastrointestinal endoscopy without signs of bleeding. Moreover, there was no evidence of gastric and esophageal varices and portal hypertensive gastropathy. Liver biopsy showed mild and focal chronic hepatitis and cholestasis without any features suggesting fibrosis or cirrhosis (Fig. 3). A diagnosis of INCPH combined with AS was made. Methotrexate and celecoxib were prescribed and our patient’s condition has been stable and uneventful, so far.
INCPH, also known as an idiopathic portal hypertension or non-cirrhotic portal fibrosis is a clinical condition secondary to increased pressure in portal vein in the absence of a known cause of liver disease or portal vein thrombosis (PVT) [1].
Although liver cirrhosis (LC) is the most frequent cause of portal hypertension, it can also develop in the absence of cirrhosis from a variety of disorders. Schistosomiasis is the most common cause. In addition, chronic liver diseases, such as non-alcoholic steatohepatitis, primary biliary cirrhosis, primary sclerosing cholangitis, congenital hepatic fibrosis, extrahepatic PVT, and Budd-Chiari syndrome, are the most common causes of non-cirrhotic portal hypertension. If all these aforementioned conditions have been rouled out, a diagnosis of INCPH can be made [6].
Both intrahepatic vascular obstruction and increased splanchnic blood flow have been suggested as responsible factors in determining portal hypertension in INCPH. An increased resistance is likely to result from the obstructed intrahepatic vessels and distorted intrahepatic angioarchitecture. Several hypotheses for the mechanisms responsible for the obliteration of portal venules have been proposed, including aberrant coagulation activation or thrombosis, acquired or inherited disorders of vascular remodeling, and endothelial injury from the immune cells [7]. An overproduction of nitric oxide, which is liberated in the sinus lining spleen cells, has been designated to be responsible for the dilatation of splenic sinuses. In patients with a more advanced disease, it is possible for increased intrahepatic resistance, which results from the obliteration of portal venous microcirculation, to lead to a further elevation of portal hypertension. It has also been speculated that increased production of endothelin-1 is likely to increase vascular resistance and stimulate periportal collagen production [6].
The etiology of INCPH includes the following: Chronic infections, exposure to medication or toxins, genetic disorders, and immunologic disorders. INCPH is frequently associated with immunological disorders, including SS, RA, and SLE. In patients with SS, a fibrogenetic process has been suggested to be an etiological factor. In patients with SLE, immunoglobulin interference with prostaglandin formation has been discovered to increase microthrombosis vulnerability. Another immunological disorder associated with INCPH is the anticardiolipin (aCL) antibody, in which its elevation can lead to the obliteration of small vessels. Primary hypogammaglobulinemia is also found in 70% of INCPH patients. Although INCPH associated with AS has not been reported to date, the relationship between INCPH and AS could be considered in that the presence of immunoglobulin G aCL antibody is higher in patients with AS than in the normal population [8].
Another speculative pathophysiology of INCPH is venopathy. Obliterative portal venopathy is a characteristic morphologic feature in INCPH, leading to an increased intra-hepatic resistance. AS has been reported to be associated with accelerated atherosclerosis including coronary artery disease [9], so speculatively has the potential to initiate the obliterative portal venopathy.
It usually presents with symptoms or signs of gastroesophageal varices, ascites, encephalopathy, splenomegaly, or hyperplenism. Moreover, the liver function is usually preserved. In over 75% of INCPH patients, varices were discovered at the initial endoscopy. The 1-year probability of developing small and large varices was 10% and 13% respectively [2]. Splenomegaly was presented in the majority of Japanese and Western patients, whereas only a minority (14%) was presented with splenomegaly in a large Indian study [6]. Splenomegaly and varices were not found in our patient.
INCPHis a clinical diagnosis, and no laboratory test has been discovered to confirm INCPH. This patient had a clinical sign of portal hypertension, such as perisplenic varices, but no history of chronic liver disease or any other conditions that may cause cirrhosis. Doppler ultrasound that shows patent portal and hepatic veins, as well as a liver biopsy that shows no cirrhosis, can lead to the diagnosis of INCPH. Although splenomegaly and varices were not found in the initial workup, they should be thoroughly evaluated in the follow-up.
A long-term prognosis remains unclear. INCPH has been considered as a benign disorder; however, over the past few years, there have been reports showing INCPH patients with poor long-term outcome, developing severe liver decompensation, multiorgan failure, and even death. A recent study reported poor survival, showing overall 10-year survival rate to be 56%[10].
INCPH is a frequently unrecognized condition, and in many instances, patients are misdiagnosed as LC. The prognosis of patients with INCPH is generally better than that of those with cirrhosis; however, a small subgroup, such as INCPH associated with immunological diseases, showed a poor prognosis [2]. Therefore, it is important for clinicians to improve the understanding of its complex pathophysiology. To improve the management of INCPH, promoting the awareness of INCPH remains to be the most important factor.
REFERENCES
1. Schouten JN, Verheij J, Seijo S. Idiopathic non-cirrhotic portal hypertension: a review. Orphanet J Rare Dis. 2015; 10:67.

2. Siramolpiwat S, Seijo S, Miquel R, Berzigotti A, Garcia-Criado A, Darnell A, et al. Idiopathic portal hypertension: natural history and long-term outcome. Hepatology. 2014; 59:2276–85.

3. Hillaire S, Bonte E, Denninger MH, Casadevall N, Cadranel JF, Lebrec D, et al. Idiopathic non-cirrhotic intrahepatic portal hypertension in the West: a re-evaluation in 28 patients. Gut. 2002; 51:275–80.

4. Cazals-Hatem D, Hillaire S, Rudler M, Plessier A, Paradis V, Condat B, et al. Obliterative portal venopathy: portal hypertension is not always present at diagnosis. J Hepatol. 2011; 54:455–61.

5. Vaiphei K, Bhatia A, Sinha SK. Liver pathology in collagen vascular disorders highlighting the vascular changes within portal tracts. Indian J Pathol Microbiol. 2011; 54:25–31.

6. Schouten JN, Garcia-Pagan JC, Valla DC, Janssen HL. Idiopathic noncirrhotic portal hypertension. Hepatology. 2011; 54:1071–81.

7. Ziol M, Poirel H, Kountchou GN, Boyer O, Mohand D, Mouthon L, et al. Intrasinusoidal cytotoxic CD8+ T cells in nodular regenerative hyperplasia of the liver. Hum Pathol. 2004; 35:1241–51.

8. Juanola X, Mateo L, Domenech P, Bas J, Contreras N, Nolla JM, et al. Prevalence of antiphospholipid antibodies in patients with ankylosing spondylitis. J Rheumatol. 1995; 22:1891–3.
9. Ungprasert P, Srivali N, Kittanamongkolchai W. Risk of coronary artery disease in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann Transl Med. 2015; 3:51.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download