Abstract
Background
Otitis media with effusion (OME) is defined as middle ear effusion without acute signs of infection. OME usually resolves spontaneously; however, persistent OME may require the insertion of a ventilation tube. This study investigated risk factors for persistent OME in children who undergo ventilation tube insertion.
Methods
Children who were admitted to undergo ventilation tube insertion at Jeju National University Hospital between August 2015 and July 2016 were enrolled as the case group. Healthy children without persistent OME from August 2016 to July 2017 were enrolled as the control group. Baseline characteristics and predisposing factor data were collected using an interview questionnaire. Middle ear fluids were collected from the case group.
Results
A total of 31 patients underwent ventilation tube insertion. The mean age of the case group was 4.53 years, with a male-to-female ratio of 21:10. Twenty-nine (93.5%) children attended a daycare center, and 21 (67.7%) had experience with bottle feeding. Fifteen (48.4%) children in the case group and 3 (9.7%) in the control group first attended a daycare center at <1 year of age (odds ratio=9.96; 95% confidence interval=2.44-39.70; p=0.001). No bacteria were found in middle ear fluid collected from the 31 operated children. Nasopharyngeal bacterial colonization was found in 13 (41.9%) and 17 (54.8%) children in the case and control groups, respectively.
Otitis media with effusion (OME) is characterized by a nonpurulent effusion from the middle ear without symptoms such as otalgia or fever. OME is believed to follow an episode of acute otitis media or abnormal Eustachian tube function. Individuals with OME usually exhibit no specific symptoms; the disease is self-limited within approximately 3 months without specific treatment [1]; however, it does recur in 30-40% of cases [2].
Some birth cohorts have reported that >90% children experience at least one episode of OME before 4 years of age [2,3]. OME is the most common cause of acquired hearing impairment in children; permanent hearing loss related to otitis media has a prevalence of 2-35 per 10,000. Although symptoms are not severe, the highest incidence of OME occurs during a highly important period of language skill development. Thereafter, periodic hearing tests are essential to preserving hearing ability in OME patients [4].
The ultimate treatment goal for OME is to mitigate hearing loss. American guidelines recommend that physicians manage children with OME who are not at risk with watchful waiting for 3 months from the date of effusion onset without antimicrobial treatment [4,5]. However, the guidelines also recommend that clinicians offer ventilation tube insertion to children with documented hearing difficulties, structural abnormalities of the tympanic membrane or middle ear, increased risk for speech and/or language difficulties, and/or reduced quality of life in cases of OME lasting ≥3 months [5].
Predisposing factors for persistent OME that require ventilation tube insertion include: attendance at a day care center, familial history of otitis media, low socio-economic status, tobacco smoke exposure and adenoid hypertrophy [6,7]. Allergic tendency or biofilm formation of nasal colonizing bacteria is suspected to contribute to the pathogenesis of persistent OME [8-10]. Recurrent otitis media is known to be another risk factor for persistent OME. Although the pathogenesis of recurrent otitis media is multifactorial, the invasion of nasopharyngeal colonizing bacteria is believed to be related [11,12]. The most frequent pathogens colonizing the nasopharynx include Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis.
In Korea, the clinical guidelines for pediatric otitis media has been updated [13]. This domestic guideline recommends the appropriate use of antimicrobials, an indication for ventilation tube insertion, and the importance of hearing tests during treatment for OME, which were not different from other guidelines [4,13]. However, long-term antimicrobial use (>3 months) for the treatment of OME was usually found in the clinical field.
A previous domestic study investigating OME reported risk factors for children undergoing recurrent ventilation tube insertion or ventilation tube insertion for children with craniofacial abnormalities [14]. We investigated the risk factors for persistent OME in children according to need for middle ear ventilation. Our aim was to determine the relationship between the colonization of bacteria in the nasopharynx and middle ear effusion.
The present investigation was a prospective case-control study. Children <12 years of age, who were admitted for ventilation tube insertion at Jeju National University Hospital (JNUH) between August 2015 and July 2016, were enrolled as the case group. All participants were enrolled when they were definitively diagnosed with OME by two otolaryngologists. The children were scheduled to undergo middle ear ventilation for persistent OME 3 months after diagnosis, despite previous antimicrobial use. Patients with immunodeficiency disorders or craniofacial abnormalities, such as cleft palate and/or cleft lip, were excluded. However, patients who previously underwent ventilation tube insertion were included.
A group of age- and sex-matched subjects representing the controls was chosen from a group of healthy children without persistent OME, who visited the JNUH between August 2016 and July 2017. Apart from no history of persistent OME, subjects with recurrent acute otitis media were excluded. The present study was approved by the Institutional Review Board of Jeju National University Hospital (IRB No. 2015-05-006). Informed written consent was obtained from all participants before enrollment.
Baseline characteristics and predisposing factors for the case and control groups were collected using a questionnaire interview. Investigated factors included: age, sex, familial member, pattern of daycare center attendance, recent history of antimicrobial use, history of breastfeedin, exposure to tobacco smoke in the house and vaccination history. Daycare center attendance was defined as attendance at a daycare center for at least 6 months, or younger subjects who attended a daycare center when enrolled in the study. Breastfeeding was defined in children who were breastfed for at least 2 months, and was distinguished from bottle feeding. History of pneumococcal conjugate and H. influenzae type b vaccination was also examined.
Microbiological investigations of middle ear effusions and colonization of the nasopharynx were performed. After aseptic myringotomy during surgery for ventilation tube insertion, a middle ear effusion sample was obtained using a sterile calcium alginate swab. If the middle ear effusion was glue-like in consistency, 0.5mL of normal saline was instilled into the middle ear and the specimen was drawn using the same method. Nasopharyngeal aspirates were obtained from both the case and control groups. The same sterile calcium alginate swab was passed through a single anterior nostril to reach the nasopharynx and was rotated. All specimens were plated on trypticase soy agar plates containing 5% sheep blood and chocolate agar plates, and were incubated overnight at 37℃ in 5% CO2. The identification of bacteria was limited to S. pneumoniae, H. influenzae, and M. catarrhalis, which are known to be the three major pathogens for otitis media. The identification method used was a routine biochemical test using the Vitek II system(bioMerieux Vitek Inc., Durham, NC, USA).
Statistical analysis was performed using R software version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria). The relationship between the investigated risk factors and middle ear ventilation tube insertion was analyzed using the chi-square test. Multivariate logistic regression analysis was performed, and the data are expressed as odds ratio (OR) and 95% confidence interval (CI). All tests were two tailed, and p<0.05 was considered to be statistically significant.
A total of 31 patients (21 male, 10 female) underwent ventilation tube insertion. The mean age of the case group was 4.53 years (median age, 4.20 years [range, 0.95-12.00 years]); the mean age of the control group was 4.06 years (median age, 3.88 years [range, 0.74-12.22 years]). In the case group, 26 patients underwent bilateral ventilation tube insertion, and 11 underwent unilateral insertion. There were no complications after the operation. Before the operation, hearing tests, such as tympanometry or pure tone audiometry, were performed in 20 (64.5%) patients. All of them had hearing loss; more specifically, 18 (90%) had 20-40 dB hearing loss, and 2 had <20 dB hearing loss. We determined ventilation tube insertion after counselling in 11 patients who did not undergo a hearing test because the parents complained of middle ear effusion >3 months.
In the case group, 29 (93.5%) children attended a daycare center, which was more than in the control group (n=23); this difference was not statistically significant (OR=5.04; 95% CI=0.98-26.09; p=0.084) (Table 1). However, the number of subjects <1 year of age who attended a daycare center was 15 (48.4%) in the case group, which was significantly more than the number of children in the control group (n=3 [48.4%]); this difference was statistically significant (OR=9.96; 95% CI=2.44-39.70; p=0.001) (Fig. 1). There were more breastfed children in the control group (n=22) than in the case group (n=19) (p=0.592), and more bottle-fed children in the case group (n=21) than in the control group (n=18) (p=0.599). However, these feeding habits had no statistically significant influence on ventilation tube insertion for OME (Table 1). Exposure to household tobacco smoke appeared to have no significant effect on persistent OME. Regardless of whether subjects had siblings, there were no differences in the prevalence of persistent OME. In the case group, there were no siblings who underwent ventilation tube insertion.
Eighteen (58.1%) children in the case group and 16 (51.6%) in the control group were taking antibiotics for persistent OME. Contrary to expectations, no bacteria were isolated from middle ear effusions collected from those who underwent ventilation tube insertion. The positive nasopharyngeal carriage rate was 41.9%(13/31) in the case group: S. pneumoniae (n=6); H. influenzae (n=2); and M. catarrhalis (n=6). In the control group, the nasopharyngeal carriage rate was 54.8%(17/31): S. pneumoniae (n=6); H. influenzae (n=7); and M. catarrhalis (n=5) (Table 2).
This investigation was a prospective study aimed at determining risk factors for ventilation tube insertion for persistent OME that did not spontaneously improve in children. Another goal of this study was to identify the relationship between nasopharyngeal colonizing bacteria and persistent middle ear effusion. Two otolaryngologists with experience in ventilation tube insertion in the middle ear were involved in this study. Indications for ventilation tube insertion were significant hearing loss due to persistent middle ear effusion, and persistent OME >3 months, which was refractory to spontaneous improvement despite of the use of antibiotics.
OME may occur after an upper respiratory tract infection due to poor Eustachian tube function or as a result of recurrent acute otitis media. A major risk factor for developing OME is age because of its direct correlation with the angulation of the Eustachian tube. Other factors reported to increase the risk for developing OME include daycare center attendance, exposure to tobacco smoke, low socio-economic status, shorter duration of breastfeeding, adenoid hypertrophy, and recurrent otitis media [6,7,15,16].
In our study, children <1 year of age, who attended a daycare center, was the only statistically significant risk factor (Fig 1). Usually, younger children had more respiratory tract infections than adults because of their immature immune system. In addition, daycare center attendance enhances close contact with other children in confined spaces. In the present study, however, daycare attendance regardless of age had no clinically significant association with persistent OME. We speculate that the difference is the diluted result because of high attendance rates at daycare centers in both the case and control groups (52/62 [83.9%]). We did not use data for recurrent otitis media as a risk factor for persistent OME. The recall ability of the patients’ parents regarding the number of episodes or treatment of otitis media was uncertain. Attendance at a daycare center, however, was easy to recall, and this factor was believed to be related to recurrent otitis media. We used this particular questionnaire for our investigation based on previous studies [17,18].
Exposure to household tobacco smoke and breastfeeding were not significant factors for persistent OME. We had no method to quantitatively evaluate the level of smoke exposure or breastfeeding. There was no relationship between bottle feeding and persistent OME. We speculate that this can be explained by the fact that most of our participants combined breast and bottle feeding.
Experts have recommended the use of pneumatic otoscopy or tympanometry to assess OME with hearing loss [4]. Furthermore, the first-line treatment choice to manage persistent OME in individuals not at risk is watchful waiting for 3 months from the date of effusion onset. This recommendation was applied to children with hearing loss <20 dB. Ventilation tube insertion is the definitive treatment for removing middle ear effusions and an effective method to prevent conductive hearing loss caused by middle ear effusion. Operative methods, such as ventilation tube insertion, are especially recommended when structural damage to the tympanic membrane, tympanosclerosis, cholesteatoma, symptoms of otalgia or vertigo, and hearing loss >40 dB, are found [4,19].
Previous studies have reported that bacterial prevalence in middle ear effusions is 20-50%, although the population was diagnosed as acute otitis media or OME [20-23]. We assumed that some bacteria could be expected to be found in middle ear effusions, and the pathogen could have a relationship with nasopharyngeal colonizing bacteria. However, no bacteria were isolated from any of the 31 middle ear effusion samples collected. This may be explained by the indication for ventilation tube insertion that was applied to the study population, which was different from other studies. Regardless of antimicrobial use in the recent 2 months, the sterile nature of middle ear effusions support no need for antibiotics to treat persistent OME. The American clinical guidelines also made strong recommendations against using systemic antibiotics for treatment OME in children [4].
Recently, molecular genetic techniques, such as polymerase chain reaction (PCR), have replaced conventional culture methods. Using these recently developed techniques, detection rates of causative pathogens have increased. This methodology has been applied to middle ear fluid specimens [24,25]. However, we could not perform a PCR assay due to a storage problem. In addition, considering the over-detection of inactive bacteria in PCR assays, we focused on living bacteria isolated using conventional culture methods.
The contribution of nasopharyngeal colonizing bacteria to otitis media has been established, and the bacterial pathogens had been studied [26,27]. In our study, there was no difference in antimicrobial use during a recent 2-month period between the case and control groups. However, nasopharyngeal colonization rates in the control group (54.8%) were greater than those in the case group (41.9%). This result suggests that other factors, in addition to nasopharyngeal colonizing bacteria, contribute to persistent OME. We cannot draw definitive conclusions because some patients in the control group visited our hospital complaining of respiratory symptoms.
This study had some limitations, the first of which were the relatively small sample size and its single-center design. Other predisposing factors, such as adenoid hypertrophy and allergic disorders, could not be investigated. If patients who spontaneously improved after the diagnosis of OME were investigated, risk factors for ventilation tube insertion could be more defined. Despite these limitations, this study should prompt clinicians to prospectively investigate risk factors for ventilation tube insertion in patients with OME disease only. Additionally, during the study period, we applied strict indications for ventilation tube insertion while considering the guidelines. We were able to confirm the principle of test hearing loss watching for spontaneous improvement. The sterile middle ear fluid found in children with OME children support the efficacy of antimicrobial use. Earlier attendance at a daycare center was the only statistically significant risk factor and, therefore, should prompt careful consideration before a decision is made to send a child to a daycare center. Future studies investigating OME should further examine the relationship between ventilation tube insertion and antimicrobial use. Furthermore, we believe that molecular-based methods investigating middle ear effusion and nasopharyngeal aspirates are promising research areas to pursue.
ACKNOWLEDGMENTS
This work was supported by a research grant from Jeju National University Hospital in 2015.
REFERENCES
1. Teele DW, Klein JO, Rosner BA. Epidemiology of otitis media in children. Ann Otol Rhinol Laryngol Suppl. 1980; 89:5–6.

2. Williamson IG, Dunleavey J, Bain J, Robinson D. The natural history of otitis media with effusion--a three-year study of the incidence and prevalence of abnormal tympanograms in four South West Hampshire infant and first schools. J Laryngol Otol. 1994; 108:930–4.
3. Tos M. Epidemiology and natural history of secretory otitis. Am J Otol. 1984; 5:459–62.
4. Rosenfeld RM, Shin JJ, Schwartz SR, Coggins R, Gagnon L, Hackell JM, et al. Clinical practice guideline: otitis media with effusion (update). Otolaryngol Head Neck Surg. 2016; 154(1 Suppl):S1–41.

5. Rosenfeld RM, Schwartz SR, Pynnonen MA, Tunkel DE, Hussey HM, Fichera JS, et al. Clinical practice guideline: Tympanostomy tubes in children. Otolaryngol Head Neck Surg. 2013; 149(1 Suppl):S1–35.
6. Williamson I. Otitis media with effusion in children. BMJ Clin Evid. 2011; 2011:pii: 0502.
7. Owen MJ, Baldwin CD, Swank PR, Pannu AK, Johnson DL, Howie VM. Relation of infant feeding practices, cigarette smoke exposure, and group child care to the onset and duration of otitis media with effusion in the first two years of life. J Pediatr. 1993; 123:702–11.

8. Alles R, Parikh A, Hawk L, Darby Y, Romero JN, Scadding G. The prevalence of atopic disorders in children with chronic otitis media with effusion. Pediatr Allergy Immunol. 2001; 12:102–6.

9. Gok U, Bulut Y, Keles E, Yalcin S, Doymaz MZ. Bacteriological and PCR analysis of clinical material aspirated from otitis media with effusions. Int J Pediatr Otorhinolaryngol. 2001; 60:49–54.

10. Kreiner-Møller E, Chawes BL, Caye-Thomasen P, Bønnelykke K, Bisgaard H. Allergic rhinitis is associated with otitis media with effusion: a birth cohort study. Clin Exp Allergy. 2012; 42:1615–20.

11. Faden H, Duffy L, Wasielewski R, Wolf J, Krystofik D, Tung Y. Relationship between nasopharyngeal colonization and the development of otitis media in children. J Infect Dis. 1997; 175:1440–5.

12. García-Rodríguez JA, Fresnadillo Martínez MJ. Dynamics of nasopharyngeal colonization by potential respiratory pathogens. J Antimicrob Chemother. 2002; 50(Suppl S2):59–73.

13. Korean Otologic Society. Korean clinical practive guideline: otitis media in children 2014 [Internet]. Seoul: Korean Medical Guideline Information Center;2014. [cited 2018 April 3]. http://www.otologicalsociety.or.kr.
14. Ahn JH, Yoon TH, Pae KH, Kim TS, Chung JW, Lee KS. Clinical manifestations and risk factors of children receiving triple ventilating tube insertions for treatment of recurrent otitis media with effusion. Pediatrics. 2006; 117:e1119. –23.

15. Pestalozza G, Romagnoli M, Tessitore E. Incidence and risk factors of acute otitis media and otitis media with effusion in children of different age groups. Adv Otorhinolaryngol. 1988; 40:47–56.

16. Casselbrant ML, Mandel EM, Doyle WJ. Information on comorbidities collected by history is useful for assigning Otitis Media risk to children. Int J Pediatr Otorhinolaryngol. 2016; 85:136–40.

17. Kim EJ, Kwon YR, Song KH, Jang WN, Lee J, Chang JK, et al. A study on risk factors of recurrent otitis media. Korean J Pediatr Infect Dis. 2010; 17:91–100. Korean.

18. Rovers MM, Zielhuis GA, Ingels K, van der Wilt GJ. Day-care and otitis media in young children: a critical overview. Eur J Pediatr. 1999; 158:1–6.
19. Khanna R, Lakhanpaul M, Bull PD; Guideline Development Group. Surgical management of otitis media with effusion in children: summary of NICE guidance. Clin Otolaryngol. 2008; 33:600–5.

20. Poetker DM, Lindstrom DR, Edmiston CE, Krepel CJ, Link TR, Kerschner JE. Microbiology of middle ear effusions from 292 patients undergoing tympanostomy tube placement for middle ear disease. Int J Pediatr Otorhinolaryngol. 2005; 69:799–804.

21. Kim SH, Jeon EJ, Hong SM, Bae CH, Lee HY, Park MK, et al. Bacterial species and antibiotic sensitivity in korean patients diagnosed with acute otitis media and otitis media with effusion. J Korean Med Sci. 2017; 32:672–8.

22. Holder RC, Kirse DJ, Evans AK, Peters TR, Poehling KA, Swords WE, et al. One third of middle ear effusions from children undergoing tympanostomy tube placement had multiple bacterial pathogens. BMC Pediatr. 2012; 12:87.

23. Ngo CC, Massa HM, Thornton RB, Cripps AW. Predominant bacteria detected from the middle ear fluid of children experiencing otitis media: a systematic review. PLoS One. 2016; 11:e0150949.

24. Farajzadah Sheikh A, Saki N, Roointan M, Ranjbar R, Yadyad MJ, Kaydani A, et al. Identification of Alloiococcus otitidis, Streptococcus pneumoniae, Moraxella catarrhalis and Haemophilus influenzae in children with otitis media with effusion. Jundishapur J Microbiol. 2015; 8:e17985.

25. Jervis-Bardy J, Carney AS, Duguid R, Leach AJ. Microbiology of otitis media in Indigenous Australian children: review. J Laryngol Otol. 2017; 131(S2):S2–11.

Fig. 1.
Odds ratio of patient characteristics who underwent ventilation tube insertion. DCC, daycare center; Hib, Haemophilus influenzae type b; PCV, pneumococcal conjugate vaccine.
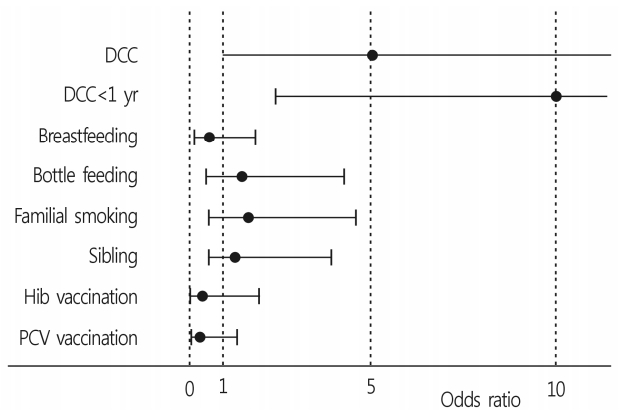
Table 1.
General characteristics of otitis media in the case and control groups
| Variable | Case group (n=31) | Control group (n=31) | ORa) (95% CI) | p-value |
|---|---|---|---|---|
| Number (%) | Number (%) | |||
| Gender (male:female) | 21:10 | 21:10 | ||
| Mean age (yr) (range) | 4.53 (0.95-12.00) | 4.06 (0.74-12.22) | ||
| Daycare center | 29 (93.5) | 23 (74.2) | 5.04 (0.98-26.09) | 0.084 |
| <1 yrb) | 15 (48.4) | 3 (9.7) | 9.96 (2.44-39.70) | 0.001 |
| Breast feeding | 19 (61.3) | 22 (71.0) | 0.64 (0.22-1.87) | 0.592 |
| Bottle feeding | 21 (67.7) | 18 (58.1) | 1.51 (0.53-4.27) | 0.599 |
| Familial smoking | 18 (58.1) | 14 (45.2) | 1.68 (0.62-4.59) | 0.445 |
| Sibling | 22 (71.0) | 20 (64.5) | 1.34 (0.61-3.91) | 0.785 |
| Hib vaccination | 25 (80.6) | 28 (90.3) | 0.45 (0.10-1.97) | 0.471 |
| PCV vaccination | 20 (64.5) | 25 (80.6) | 0.43 (0.13-1.38) | 0.254 |
Table 2.
Microbiological status of otitis media in the case and control groups
| Microbiologic status | Case | Control |
|---|---|---|
| Middle ear fluid | 0 (0.0) | NAa) |
| Nasopharyngeal aspirate | 13 (41.9) | 17 (54.8) |
| S. pneumoniae | 6 (19.4) | 6 (19.4) |
| H. influenzae | 2 (6.5) | 7 (22.6) |
| M. catarrhalis | 6 (19.4) | 5 (16.1) |
| Previous antibioticsb) | 18 (58.1) | 16 (51.6) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download