1. Ellison DH, Felker GM. Diuretic treatment in heart failure. N Engl J Med. 2017; 377:1964–1975. PMID:
29141174.

2. Benedict CR, Weiner DH, Johnstone DE, et al. Comparative neurohormonal responses in patients with preserved and impaired left ventricular ejection fraction: results of the Studies of Left Ventricular Dysfunction (SOLVD) registry. J Am Coll Cardiol. 1993; 22:146A–153A.
3. Benedict CR, Johnstone DE, Weiner DH, et al. Relation of neurohumoral activation to clinical variables and degree of ventricular dysfunction: a report from the Registry of Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1994; 23:1410–1420. PMID:
7909822.

4. Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999; 341:577–585. PMID:
10451464.

5. Iwata M, Cowling RT, Yeo SJ, Greenberg B. Targeting the ACE2-Ang-(1-7) pathway in cardiac fibroblasts to treat cardiac remodeling and heart failure. J Mol Cell Cardiol. 2011; 51:542–547. PMID:
21147120.

6. Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000; 35:569–582. PMID:
10716457.

7. Carley AN, Taegtmeyer H, Lewandowski ED. Matrix revisited: mechanisms linking energy substrate metabolism to the function of the heart. Circ Res. 2014; 114:717–729. PMID:
24526677.
8. Heggermont WA, Papageorgiou AP, Heymans S, van Bilsen M. Metabolic support for the heart: complementary therapy for heart failure? Eur J Heart Fail. 2016; 18:1420–1429. PMID:
27813339.

9. Kim SY, Morales CR, Gillette TG, Hill JA. Epigenetic regulation in heart failure. Curr Opin Cardiol. 2016; 31:255–265. PMID:
27022893.

10. Murphy SP, Kakkar R, McCarthy CP, Januzzi JL Jr. Inflammation in heart failure: JACC state-of-the-art review. J Am Coll Cardiol. 2020; 75:1324–1340. PMID:
32192660.
11. Ferrario CM, Ahmad S, Varagic J, et al. Intracrine angiotensin II functions originate from noncanonical pathways in the human heart. Am J Physiol Heart Circ Physiol. 2016; 311:H404–H414. PMID:
27233763.

12. Pfeffer JM, Pfeffer MA, Mirsky I, Braunwald E. Regression of left ventricular hypertrophy and prevention of left ventricular dysfunction by captopril in the spontaneously hypertensive rat. Proc Natl Acad Sci U S A. 1982; 79:3310–3314. PMID:
6212929.

13. Pfeffer MA, Braunwald E, Moyé LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. N Engl J Med. 1992; 327:669–677. PMID:
1386652.

14. St John Sutton M, Pfeffer MA, Moye L, et al. Cardiovascular death and left ventricular remodeling two years after myocardial infarction: baseline predictors and impact of long-term use of captopril: information from the Survival and Ventricular Enlargement (SAVE) trial. Circulation. 1997; 96:3294–3299. PMID:
9396419.

15. St John Sutton M, Pfeffer MA, Plappert T, et al. Quantitative two-dimensional echocardiographic measurements are major predictors of adverse cardiovascular events after acute myocardial infarction. The protective effects of captopril. Circulation. 1994; 89:68–75. PMID:
8281697.

16. CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987; 316:1429–1435. PMID:
2883575.
17. SOLVD Investigators. Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991; 325:293–302. PMID:
2057034.

18. SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB Jr, Cohn JN. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992; 327:685–691. PMID:
1463530.

19. Greenberg B, Quinones MA, Koilpillai C, et al. Effects of long-term enalapril therapy on cardiac structure and function in patients with left ventricular dysfunction. Results of the SOLVD echocardiography substudy. Circulation. 1995; 91:2573–2581. PMID:
7743619.

20. Konstam MA, Kronenberg MW, Rousseau MF, et al. Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dilatation in patients with asymptomatic systolic dysfunction. Circulation. 1993; 88:2277–2283. PMID:
8222122.

21. Quiñones MA, Greenberg BH, Kopelen HA, et al. Echocardiographic predictors of clinical outcome in patients with left ventricular dysfunction enrolled in the SOLVD registry and trials: significance of left ventricular hypertrophy. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 2000; 35:1237–1244. PMID:
10758966.

22. Garg R, Yusuf S. Collaborative Group on ACE Inhibitor Trials. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. JAMA. 1995; 273:1450–1456. PMID:
7654275.

23. Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003; 362:772–776. PMID:
13678870.

24. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013; 62:e147–e239. PMID:
23747642.
25. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017; 70:776–803. PMID:
28461007.

26. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42:3599–3726. PMID:
34447992.
27. Writing Committee, Maddox TM, Januzzi JL, et al. 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021; 77:772–810. PMID:
33446410.

28. D’Elia E, Iacovoni A, Vaduganathan M, Lorini FL, Perlini S, Senni M. Neprilysin inhibition in heart failure: mechanisms and substrates beyond modulating natriuretic peptides. Eur J Heart Fail. 2017; 19:710–717. PMID:
28326642.

29. McClean DR, Ikram H, Mehta S, et al. Vasopeptidase inhibition with omapatrilat in chronic heart failure: acute and long-term hemodynamic and neurohumoral effects. J Am Coll Cardiol. 2002; 39:2034–2041. PMID:
12084605.

30. Packer M, Califf RM, Konstam MA, et al. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE). Circulation. 2002; 106:920–926. PMID:
12186794.

31. McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014; 371:993–1004. PMID:
25176015.

32. Desai AS, McMurray JJ, Packer M, et al. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J. 2015; 36:1990–1997. PMID:
26022006.

33. Vardeny O, Claggett B, Packer M, et al. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail. 2016; 18:1228–1234. PMID:
27283779.

34. Böhm M, Young R, Jhund PS, et al. Systolic blood pressure, cardiovascular outcomes and efficacy and safety of sacubitril/valsartan (LCZ696) in patients with chronic heart failure and reduced ejection fraction: results from PARADIGM-HF. Eur Heart J. 2017; 38:1132–1143. PMID:
28158398.

35. Balmforth C, Simpson J, Shen L, et al. Outcomes and effect of treatment according to etiology in HFrEF: an analysis of PARADIGM-HF. JACC Heart Fail. 2019; 7:457–465. PMID:
31078482.
36. Jhund PS, Fu M, Bayram E, et al. Efficacy and safety of LCZ696 (sacubitril-valsartan) according to age: insights from PARADIGM-HF. Eur Heart J. 2015; 36:2576–2584. PMID:
26231885.

37. Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019; 380:539–548. PMID:
30415601.

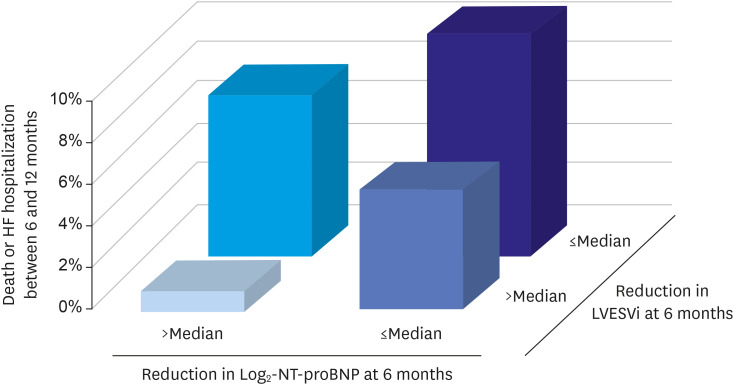
38. Januzzi JL Jr, Prescott MF, Butler J, et al. Association of change in N-Terminal pro-B-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA. 2019; 322:1085–1095. PMID:
31475295.

39. Pitt B. “Escape” of aldosterone production in patients with left ventricular dysfunction treated with an angiotensin converting enzyme inhibitor: implications for therapy. Cardiovasc Drugs Ther. 1995; 9:145–149. PMID:
7786835.

40. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999; 341:709–717. PMID:
10471456.

41. Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003; 348:1309–1321. PMID:
12668699.

42. Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011; 364:11–21. PMID:
21073363.

43. Waagstein F, Hjalmarson A, Varnauskas E, Wallentin I. Effect of chronic beta-adrenergic receptor blockade in congestive cardiomyopathy. Br Heart J. 1975; 37:1022–1036. PMID:
1191416.

44. Waagstein F, Bristow MR, Swedberg K, et al. Beneficial effects of metoprolol in idiopathic dilated cardiomyopathy. Lancet. 1993; 342:1441–1446. PMID:
7902479.

45. Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996; 334:1349–1355. PMID:
8614419.

46. Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001; 344:1651–1658. PMID:
11386263.

47. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999; 353:2001–2007. PMID:
10376614.
48. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999; 353:9–13. PMID:
10023943.
49. Udell JA, Cavender MA, Bhatt DL, Chatterjee S, Farkouh ME, Scirica BM. Glucose-lowering drugs or strategies and cardiovascular outcomes in patients with or at risk for type 2 diabetes: a meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol. 2015; 3:356–366. PMID:
25791290.

50. Control Group. Turnbull FM, Abraira C, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009; 52:2288–2298. PMID:
19655124.

51. Zelniker TA, Braunwald E. Cardiac and renal effects of sodium-glucose co-transporter 2 inhibitors in diabetes: JACC state-of-the-art review. J Am Coll Cardiol. 2018; 72:1845–1855. PMID:
30075873.

52. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; 373:2117–2128. PMID:
26378978.

53. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017; 377:644–657. PMID:
28605608.

54. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019; 380:347–357. PMID:
30415602.

55. Fitchett D, Butler J, van de Borne P, et al. Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA-REG OUTCOME
® trial. Eur Heart J. 2018; 39:363–370. PMID:
29020355.

56. Rådholm K, Figtree G, Perkovic V, et al. Canagliflozin and heart failure in type 2 diabetes mellitus: results from the CANVAS program. Circulation. 2018; 138:458–468. PMID:
29526832.

57. Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl Sci. 2020; 5:632–644. PMID:
32613148.

58. McMurray JJ, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019; 381:1995–2008. PMID:
31535829.

59. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020; 383:1413–1424. PMID:
32865377.
60. Zannad F, Ferreira JP, Pocock SJ, et al. Cardiac and kidney benefits of empagliflozin in heart failure across the spectrum of kidney function: insights from EMPEROR-Reduced. Circulation. 2021; 143:310–321. PMID:
33095032.

61. Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020; 396:819–829. PMID:
32877652.

62. Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021; 384:117–128. PMID:
33200892.

63. Tromp J, Ponikowski P, Salsali A, et al. Sodium-glucose co-transporter 2 inhibition in patients hospitalized for acute decompensated heart failure: rationale for and design of the EMPULSE trial. Eur J Heart Fail. 2021; 23:826–834. PMID:
33609072.

64. Voors AA. EMPULSE - Efficacy and safety of empagliflozin in hospitalized heart failure patients: main results from the EMPULSE trial. Presentation sessions at: The 2021 American Heart Association (AHA) 2021 Annual Meeting. 2021 Nov 13-15; Boston (MA). Dallas (TX): American Heart Association;2021.
65. Massie B, Chatterjee K, Werner J, Greenberg B, Hart R, Parmley WW. Hemodynamic advantage of combined administration of hydralazine orally and nitrates nonparenterally in the vasodilator therapy of chronic heart failure. Am J Cardiol. 1977; 40:794–801. PMID:
411364.

66. Greenberg BH, Massie BM, Brundage BH, Botvinick EH, Parmley WW, Chatterjee K. Beneficial effects of hydralazine in severe mitral regurgitation. Circulation. 1978; 58:273–279. PMID:
668075.

67. Walsh WF, Greenberg BH. Results of long-term vasodilator therapy in patients with refractory congestive heart failure. Circulation. 1981; 64:499–505. PMID:
7261282.

68. Cohn JN, Archibald DG, Ziesche S, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. results of a Veterans Administration Cooperative Study. N Engl J Med. 1986; 314:1547–1552. PMID:
3520315.

69. Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med. 1991; 325:303–310. PMID:
2057035.

70. Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004; 351:2049–2057. PMID:
15533851.

71. Kannel WB, Kannel C, Paffenbarger RS Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987; 113:1489–1494. PMID:
3591616.

72. Fox K, Ford I, Steg PG, et al. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet. 2008; 372:817–821. PMID:
18757091.

73. Stieber J, Herrmann S, Feil S, et al. The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. Proc Natl Acad Sci U S A. 2003; 100:15235–15240. PMID:
14657344.

74. Bucchi A, Tognati A, Milanesi R, Baruscotti M, DiFrancesco D. Properties of ivabradine-induced block of HCN1 and HCN4 pacemaker channels. J Physiol. 2006; 572:335–346. PMID:
16484306.

75. Thollon C, Vilaine JP. I(f) inhibition in cardiovascular diseases. Adv Pharmacol. 2010; 59:53–92. PMID:
20933199.

76. Fox K, Ford I, Steg PG, Tendera M, Ferrari R. BEAUTIFUL Investigators. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet. 2008; 372:807–816. PMID:
18757088.

77. Fox K, Ford I, Steg PG, et al. Ivabradine in stable coronary artery disease without clinical heart failure. N Engl J Med. 2014; 371:1091–1099. PMID:
25176136.

78. Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010; 376:875–885. PMID:
20801500.

79. Tanboğa İH, Topçu S, Aksakal E, et al. The risk of atrial fibrillation with ivabradine treatment: a meta-analysis with trial sequential analysis of more than 40000 patients. Clin Cardiol. 2016; 39:615–620. PMID:
27511965.

80. Derbyshire ER, Marletta MA. Biochemistry of soluble guanylate cyclase. Handb Exp Pharmacol. 2009; 191:17–31.

81. Martin E, Berka V, Tsai AL, Murad F. Soluble guanylyl cyclase: the nitric oxide receptor. Methods Enzymol. 2005; 396:478–492. PMID:
16291255.

82. Gheorghiade M, Marti CN, Sabbah HN, et al. Soluble guanylate cyclase: a potential therapeutic target for heart failure. Heart Fail Rev. 2013; 18:123–134. PMID:
22622468.

83. Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation. 2011; 123:2263–2273. PMID:
21606405.

84. Follmann M, Ackerstaff J, Redlich G, et al. Discovery of the soluble guanylate cyclase stimulator vericiguat (BAY 1021189) for the treatment of chronic heart failure. J Med Chem. 2017; 60:5146–5161. PMID:
28557445.
85. Gheorghiade M, Greene SJ, Butler J, et al. Effect of vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in patients with worsening chronic heart failure and reduced ejection fraction: the SOCRATES-REDUCED randomized trial. JAMA. 2015; 314:2251–2262. PMID:
26547357.

86. Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020; 382:1883–1893. PMID:
32222134.

87. Ezekowitz JA, O’Connor CM, Troughton RW, et al. N-terminal pro-B-type natriuretic peptide and clinical outcomes: Vericiguat Heart Failure With Reduced Ejection Fraction Study. JACC Heart Fail. 2020; 8:931–939. PMID:
33039447.
88. Withering W. An Account of the Foxglove, and Some of Its Medical Uses: With Practical Remarks on Dropsy and Other Diseases. Cambridge: Cambridge University Press;2014.
89. Rahimtoola SH. Digitalis therapy for patients in clinical heart failure. Circulation. 2004; 109:2942–2946. PMID:
15210610.

90. Young JB. Whither Withering’s Legacy? Digoxin’s role in our contemporary pharmacopeia for heart failure. J Am Coll Cardiol. 2005; 46:505–507. PMID:
16053965.
91. Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997; 336:525–533. PMID:
9036306.
92. Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumholz HM. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA. 2003; 289:871–878. PMID:
12588271.

93. Adams KF Jr, Patterson JH, Gattis WA, et al. Relationship of serum digoxin concentration to mortality and morbidity in women in the digitalis investigation group trial: a retrospective analysis. J Am Coll Cardiol. 2005; 46:497–504. PMID:
16053964.

94. Psotka MA, Gottlieb SS, Francis GS, et al. Cardiac calcitropes, myotropes, and mitotropes: JACC review topic of the week. J Am Coll Cardiol. 2019; 73:2345–2353. PMID:
31072579.
95. Maack C, Eschenhagen T, Hamdani N, et al. Treatments targeting inotropy. Eur Heart J. 2019; 40:3626–3644. PMID:
30295807.

96. Psotka MA, Teerlink JR. Direct myosin activation by omecamtiv mecarbil for heart failure with reduced ejection fraction. Handb Exp Pharmacol. 2017; 243:465–490. PMID:
28315072.

97. Malik FI, Hartman JJ, Elias KA, et al. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science. 2011; 331:1439–1443. PMID:
21415352.

98. Vu T, Ma P, Xiao JJ, Wang YM, Malik FI, Chow AT. Population pharmacokinetic-pharmacodynamic modeling of omecamtiv mecarbil, a cardiac myosin activator, in healthy volunteers and patients with stable heart failure. J Clin Pharmacol. 2015; 55:1236–1247. PMID:
25951506.

99. Greenberg BH, Chou W, Saikali KG, et al. Safety and tolerability of omecamtiv mecarbil during exercise in patients with ischemic cardiomyopathy and angina. JACC Heart Fail. 2015; 3:22–29. PMID:
25453536.

100. Teerlink JR, Felker GM, McMurray JJ, et al. Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure (COSMIC-HF): a phase 2, pharmacokinetic, randomised, placebo-controlled trial. Lancet. 2016; 388:2895–2903. PMID:
27914656.

101. Teerlink JR, Diaz R, Felker GM, et al. Omecamtiv mecarbil in chronic heart failure with reduced ejection fraction: rationale and design of GALACTIC-HF. JACC Heart Fail. 2020; 8:329–340. PMID:
32035892.

102. Teerlink JR, Diaz R, Felker GM, et al. Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med. 2021; 384:105–116. PMID:
33185990.
103. Felker GM, Solomon SD, Claggett B, et al. Assessment of omecamtiv mecarbil for the treatment of patients with severe heart failure: a post hoc analysis of data from the GALACTIC-HF randomized clinical trial. JAMA Cardiol. 2022; 7:26–34. PMID:
34643642.

104. Rincon MY, VandenDriessche T, Chuah MK. Gene therapy for cardiovascular disease: advances in vector development, targeting, and delivery for clinical translation. Cardiovasc Res. 2015; 108:4–20. PMID:
26239654.

105. Ishikawa K, Weber T, Hajjar RJ. Human cardiac gene therapy. Circ Res. 2018; 123:601–613. PMID:
30355138.

106. Hayward C, Patel H, Lyon A. Gene therapy in heart failure. SERCA2a as a therapeutic target. Circ J. 2014; 78:2577–2587. PMID:
25327883.
107. Gwathmey JK, Yerevanian A, Hajjar RJ. Targeting sarcoplasmic reticulum calcium ATPase by gene therapy. Hum Gene Ther. 2013; 24:937–947. PMID:
24164241.

108. Jaski BE, Jessup ML, Mancini DM, et al. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009; 15:171–181. PMID:
19327618.

109. Jessup M, Greenberg B, Mancini D, et al. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca
2+-ATPase in patients with advanced heart failure. Circulation. 2011; 124:304–313. PMID:
21709064.

110. Greenberg B, Butler J, Felker GM, et al. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Patients with Cardiac Disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet. 2016; 387:1178–1186. PMID:
26803443.

111. Rowland TJ, Sweet ME, Mestroni L, Taylor MR. Danon disease - dysregulation of autophagy in a multisystem disorder with cardiomyopathy. J Cell Sci. 2016; 129:2135–2143. PMID:
27165304.

112. Taylor MRG, Adler ED. Danon disease. Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Mirzaa GM, Amemiya A, editors. GeneReviews
® [Internet]. Seattle (WA): University of Washington, Seattle;1993-2021. cited 2020 March 5. Available from:
https://www.ncbi.nlm.nih.gov/books/NBK554742/.
113. Greenberg B. Results from First-In-Human Clinical Trial Of RP-A501 (AAV9:LAMP2B) gene therapy treatment for Danon disease. In : Presented at Heart Failure Society of America (HFSA) Annual Scientific Meeting 2021 (HFSA 2021); 2021 Sep 10-13; Aurora (CO). Rockville (MD): Heart Failure Society of America;2021.
114. Perin EC. DREAM-HF - Randomized trial of targeted transendocardial delivery of mesenchymal precursor cells in high-risk chronic heart failure patients with reduced ejection fraction. Presentation sessions at: The 2021 American Heart Association (AHA) 2021 Annual Meeting. 2021 Nov 13-15; Boston (MA). Dallas (TX): American Heart Association;2021.
115. Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF registry. J Am Coll Cardiol. 2018; 72:351–366. PMID:
30025570.
116. Greene SJ, Fonarow GC, DeVore AD, et al. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019; 73:2365–2383. PMID:
30844480.

117. Packer M, Poole-Wilson PA, Armstrong PW, et al. Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. Circulation. 1999; 100:2312–2318. PMID:
10587334.

118. Bristow MR, Gilbert EM, Abraham WT, et al. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. Circulation. 1996; 94:2807–2816. PMID:
8941106.

119. Bristow MR, O’Connell JB, Gilbert EM, et al. Dose-response of chronic beta-blocker treatment in heart failure from either idiopathic dilated or ischemic cardiomyopathy. Circulation. 1994; 89:1632–1642. PMID:
7908610.

120. Vardeny O, Claggett B, Packer M, et al. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail. 2016; 18:1228–1234. PMID:
27283779.

121. Jhund PS, Fu M, Bayram E, et al. Efficacy and safety of LCZ696 (sacubitril-valsartan) according to age: insights from PARADIGM-HF. Eur Heart J. 2015; 36:2576–2584. PMID:
26231885.

122. Seo WW, Park JJ, Park HA, et al. Guideline-directed medical therapy in elderly patients with heart failure with reduced ejection fraction: a cohort study. BMJ Open. 2020; 10:e030514.

123. Burnett H, Earley A, Voors AA, et al. Thirty years of evidence on the efficacy of drug treatments for chronic heart failure with reduced ejection fraction: a network meta-analysis. Circ Heart Fail. 2017; 10:e003529. PMID:
28087688.









 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download