Chronic total occlusion (CTO) lesion is the most challenging and rapidly evolving area in the field of percutaneous coronary intervention (PCI) in recent years. Although there are differences in the studies, CTOs are considerably observed among patients undergoing coronary angiography and CTO PCI has been substantially increasing state based on the improved success rates of CTO PCI using advanced techniques and equipment.1)2)
Despite improved success rate and observational studies’ favorable results, current randomized controlled trial (RCT) for the CTO PCI did not show improved mortality and myocardial infarction, but may improve quality of life.3) So, it is essential to choose the appropriate CTO patients for revascularization. The current guideline recommended “Percutaneous recanalization of CTOs should be considered in patients with angina resistant to medical therapy or with large area of documented ischemia in the territory of the occluded vessel.”4) Considering that angina symptoms are subjective and sham-controlled CTO PCI study related to improvement of quality of life is absent, proof of objective ischemia could be initial step for CTO PCI.
Recent several observational studies and 1 RCT showed that CTO PCI improved myocardial perfusion and reduced ischemic burden (Table 1).5)6)7)8) These data based on the ischemic burden reduction after CTO PCI make us expect improved angina symptom as well as lower rates of all-cause death and myocardial infarction at long-term follow-up, while previous RCTs didn’t assess ischemic burden.
However, studies mentioned above left something to be desired. the IMPACTOR-CTO trial5) and other studies6)7)8) enrolled limited to right coronary artery (RCA) CTO lesion or more than 70% RCA lesion. The territory of RCA has relatively limited ischemic burden compared with that of left anterior descending artery (LAD) with greater amount of myocardium at risk, so prognostic values relatively cannot help limitation. We need study that has well balanced CTO lesion and larger ischemic burden such as LAD CTO. In addition, multi-vessel disease was enrolled more than 20% in the above studies. Ischemic burden for CTO of multi-vessel disease could be vague in the physiologic assessment.
In this issue of Korean Circulation Journal, Yoon et al.9) evaluated the impact of “Ischemic burden assessment using single photon emission computed tomography in single vessel chronic total occlusion of coronary artery.” A total of 165 patients with symptomatic angina underwent CTO PCI and single photon emission computed tomography (SPECT) was analyzed. SPECT results between different CTO groups were compared. Overall prevalence and predictors for high-ischemic burden (>10%) was analyzed.
The target CTO vessels are relatively well distributed. LAD, left circumflex coronary artery (LCX), and RCA CTO are 56.4%, 10.9%, 32.7%, respectively. The median ischemic burden of LAD CTO was 10.3%. Especially, the median value of the ischemic burden of proximal LAD CTO was 14.7%. In contrast, the median ischemic burden of LCX and RCA are 5.9% and 5.9%. There was no significant relationship between the lesion location and ischemic burden in the LCX and RCA CTOs. There was no difference in long-term clinical outcomes according to ischemic burden (high >10% and low ≤10%) at baseline SPECT result in the patients with successful CTO PCI.
In summary, only 40% of patients with single vessel CTO had ischemic burden >10%. Predictors for high-ischemic burden were hypertension, baseline left ventricular ejection fraction (LVEF) >45%, LAD CTO, proximal CTO location, and de novo CTO.
The interesting finding or strength of current study is that patients with high ischemic burden were limited among successful CTO PCI in the real practice. Despite current more attempt and higher success rate of CTO PCI, this study result might explain why previous RCTs did not show better prognosis for the hard clinical outcomes such as mortality, myocardial infarction compared with optimal medical therapy. It also showed why we should assess the ischemic burden before performing CTO PCI if patients don’t have definite angina symptom or ischemia. Another is that LAD CTO lesion meaning relatively high ischemic burden was enrolled more than 55%. The above mentioned studies assessed the ischemic burden but deviated to RCA. However, current study showed well balanced lesion location. Lastly, degree of ischemic burden was objectively measured because patients with single CTO enrolled. In the real practice, multi-vessel disease including CTO vessel is more observed but it can make us confusion in the assessment of ischemic burden. In the setting of study, clear cut-point assessing single vessel CTO is better in the interpretation of results.
From current study results, we can derive the clinical relevance following below: before performing CTO PCI, if possible, physiologic assessment might be helpful for appropriate patient selection and then CT angiography would be performed for coronary anatomy in patients with high CTO lesion score. However, proximal LAD CTO of patients with preserved LV systolic function should be considered for revascularization even though absence of ischemic burden assessment. Conversely, non-LAD CTO of patient with ISR and reduced LVEF might bring less clinical benefit from PCI.
Recent ISCHEMIA trial analysis10) supports this approach for CTO lesion. This analysis showed ischemia severity was not associated with increased risk after adjustment for CAD severity. Concretely, 2 or 3 vessel disease including proximal LAD was associated with all-cause mortality and myocardial infarction. The invasive strategy for theses lesions lowered 4-year rate of cardiovascular death or MI. Although there were some differences with CTO patients, considering we generally perform CTO PCI in the stable setting, we might apply this analysis patients with CTO and decide to perform PCI or not.
This observational study has some limitations. There was no comparison of clinical outcomes between successful and failed CTO with high ischemic burden. Although angina symptom improvement is primary indication of CTO PCI, as revascularization strategy including CTO lesion ultimately pursue improved clinical outcomes, we still need to compare clinical outcomes between successful and failed CTO PCI in patient with high ischemic burden. Another is, as authors mentioned in the limitations, the possibility of selection bias of observation data.
Interventionists have been favoring CTO PCI in the current intervention era, but many physicians still can be challenging for CTO PCI in the aspect of benefit aside symptom. The dedicated CTO PCI trial including high ischemic burden as well as balanced lesion location will clarify the benefit of CTO PCI.
Notes
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
References
1. Walsh SJ, Cosgrove C, Spratt JC, Hanratty CG. A technical focus on antegrade dissection and re-entry for coronary chronic total occlusions: a practice update for 2019. Korean Circ J. 2019; 49:559–567. PMID: 31243929.

2. Dash D. Iteration of reverse controlled antegrade and retrograde tracking for coronary chronic total occlusion intervention: a current appraisal. Korean Circ J. 2020; 50:867–879. PMID: 32725995.

3. Lee SW, Lee PH, Ahn JM, et al. Randomized trial evaluating percutaneous coronary intervention for the treatment of chronic total occlusion. Circulation. 2019; 139:1674–1683. PMID: 30813758.

4. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019; 40:87–165. PMID: 30165437.
5. Obedinskiy AA, Kretov EI, Boukhris M, et al. The IMPACTOR-CTO trial. JACC Cardiovasc Interv. 2018; 11:1309–1311. PMID: 29976368.

6. Safley DM, Koshy S, Grantham JA, et al. Changes in myocardial ischemic burden following percutaneous coronary intervention of chronic total occlusions. Catheter Cardiovasc Interv. 2011; 78:337–343. PMID: 21413136.

7. Stuijfzand WJ, Biesbroek PS, Raijmakers PG, et al. Effects of successful percutaneous coronary intervention of chronic total occlusions on myocardial perfusion and left ventricular function. EuroIntervention. 2017; 13:345–354. PMID: 28242588.

8. Schumacher SP, Stuijfzand WJ, de Winter RW, et al. Ischemic burden reduction and long-term clinical outcomes after chronic total occlusion percutaneous coronary intervention. JACC Cardiovasc Interv. 2021; 14:1407–1418. PMID: 34238551.

9. Yoon YH, Han SW, Kwon OS, et al. Ischemic burden assessment using single photon emission computed tomography in single vessel chronic total occlusion of coronary artery. Korean Circ J. 2022; 52:150–161.

10. Reynolds HR, Shaw LJ, Min JK, et al. Outcomes in the ISCHEMIA trial based on coronary artery disease and ischemia severity. Circulation. 2021; 144:1024–1038. PMID: 34496632.

Table 1
Recent CTO PCI studies assessing ischemic burden
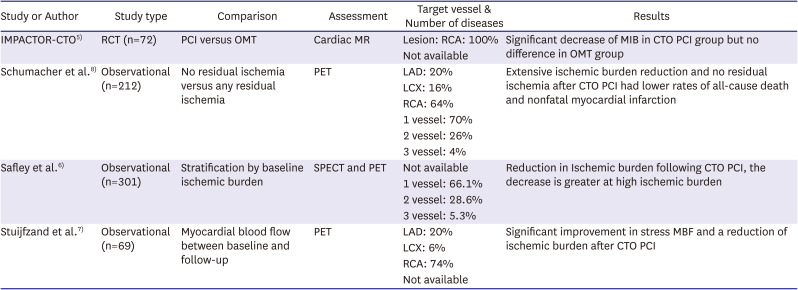
| Study or Author | Study type | Comparison | Assessment | Target vessel & Number of diseases | Results |
|---|---|---|---|---|---|
| IMPACTOR-CTO5) | RCT (n=72) | PCI versus OMT | Cardiac MR | Lesion: RCA: 100% | Significant decrease of MIB in CTO PCI group but no difference in OMT group |
| Not available | |||||
| Schumacher et al.8) | Observational (n=212) | No residual ischemia versus any residual ischemia | PET | LAD: 20% | Extensive ischemic burden reduction and no residual ischemia after CTO PCI had lower rates of all-cause death and nonfatal myocardial infarction |
| LCX: 16% | |||||
| RCA: 64% | |||||
| 1 vessel: 70% | |||||
| 2 vessel: 26% | |||||
| 3 vessel: 4% | |||||
| Safley et al.6) | Observational (n=301) | Stratification by baseline ischemic burden | SPECT and PET | Not available | Reduction in Ischemic burden following CTO PCI, the decrease is greater at high ischemic burden |
| 1 vessel: 66.1% | |||||
| 2 vessel: 28.6% | |||||
| 3 vessel: 5.3% | |||||
| Stuijfzand et al.7) | Observational (n=69) | Myocardial blood flow between baseline and follow-up | PET | LAD: 20% | Significant improvement in stress MBF and a reduction of ischemic burden after CTO PCI |
| LCX: 6% | |||||
| RCA: 74% | |||||
| Not available |
CTO = chronic total occlusion; LAD = left anterior descending artery; LCX = left circumflex coronary artery; MBF = myocardial blood flow; MIB = myocardial ischaemic burden; MR = magnetic resonance; OMT = optimal medical therapy; PCI = percutaneous coronary intervention; PET = positron emission tomography; RCA = right coronary artery; RCT = randomized controlled trial; SPECT = single photon emission computed tomography.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download