This article has been
cited by other articles in ScienceCentral.
Author's summary
Two prototypes of material to constitute surgical bioprosthetic aortic valve (AV) are bovine pericardium and porcine valves. Earlier studies have consistently shown superior hemodynamic profiles in bovine pericardial valves, however, it is not clear whether such superior hemodynamic profiles can be translated into improved clinical outcomes. In patients undergoing isolated bioprosthetic surgical aortic valve replacement (AVR) (636 patients between January 2000 and May 2016), the use of bovine pericardial valves was associated with superior hemodynamic profiles and improved late survival as compared with porcine valves. Freedom from adverse valve-related complication rates were not significantly different between the 2 groups.
Abstract
Background and Objectives
There still are controversies on which type between bovine pericardial and porcine valves is superior in the setting of aortic valve replacement (AVR). This study aims to compare clinical outcomes of AVR using between pericardial or porcine valves.
Methods
The study involved consecutive 636 patients underwent isolated AVR using stented bioprosthetic valves between January 2000 and May 2016. Of these, pericardial and porcine valves were implanted in 410 (pericardial group) and 226 patients (porcine group), respectively. Clinical outcomes including survival, structural valve deterioration (SVD) and trans-valvular pressure gradient were compared between the groups. To adjust for potential selection bias, inverse probability treatment weighting (IPTW) was conducted.
Results
The mean follow-up duration was 60.1±50.2 months. There were no significant differences in the rates of early mortality (3.1% vs. 3.1%; p=0.81) and SVD (0.3%/patient-year [PY] vs. 0.5%/PY; p=0.33) between groups. After adjustment using IPTW, however, landmark mortality analyses showed a significantly lower late (>8 years) mortality risk in pericardial group over porcine group (hazard ratio [HR], 0.61; 95% confidence interval, [CI] 0.41–0.90; p=0.01) while the risks of SVD were not significantly difference between groups (HR, 0.45; 95% CI, 0.12–1.70; p=0.24). Mean pressure gradient across prosthetic AV was lower in the Pericardial group than the Porcine group at both immediate postoperative point and latest follow-up (p values <0.001).
Conclusions
In patients undergoing bioprosthetic surgical AVR, bovine pericardial valves showed superior results in terms of postoperative hemodynamic profiles and late survival rates over porcine valves.
Keywords: Aortic valve, Bioprosthesis
INTRODUCTION
In the era of emerging trans-catheter therapy in the treatment of severe aortic valve (AV), aortic stenosis, surgical AV replacement (SAVR) still remains as the mainstay of therapy to cover a range of AV diseases.
1) In the choice of prosthetic types in heart valve replacement, bioprosthetic valves are generally preferred over mechanical prostheses in older individuals because they usually do not require lifelong anticoagulation, and exhibit lower associated risks of thromboembolism and anticoagulation-related hemorrhage while the durability of the valves may cover their life expectancy.
2)3)4) Two prototypes of material to constitute surgical bioprosthetic AV are bovine pericardium and porcine valves, the former being introduced later in clinical uses. Earlier studies have consistently shown superior hemodynamic profiles in bovine pericardial valves as compared with porcine valves demonstrating lower trans-valvular pressure gradient and larger effective orifice area.
5)6)7)8) There still are controversies, however, whether such superior hemodynamic profiles of the bovine pericardial valves may be translated into superior valve durability and improved clinical outcomes than the porcine valves in the long-term. This issue has also potential implication in the field of transcatheter aortic valve replacement (TAVR) where the prostheses consist of bovine or porcine pericardium, and studying on comparative durability of these material over porcine valves is expected to offer in-depth understanding on current TAVR valves.
To date, the data on comparative studies are still inconclusive.
9)10)11)12) In the aim of contributing to the body of evidence on this issue, we sought to compare the long-term clinical outcomes between the bovine pericardial or porcine bioprosthetic SAVR.
METHODS
Ethical statement
This study was reviewed and approved by the Asan Medical Center Institutional Ethics Committee/Review Board(study number: 2019-0467), which waived the requirement for informed patient consent because of the retrospective nature of the study.
Patients
We identified 757 adult patients (18 years or older) who underwent SAVR with bioprosthetic valves from January 2000 through May 2016 from the institutional cardiac surgery database of Asan Medical Center (Seoul, Korea). Exclusion criteria included history of prior cardiac surgery and concomitant cardiac procedures other than ablation of atrial fibrillation, atrial septal defect closure, or ascending aortic replacement to form a relatively homogeneous population while eliminating potential confounding effects from heart valve replacement/repair in other positions. As a result, a total of 636 patients who underwent SAVR with bioprosthetic valve were finally enrolled. Among them, 226 patients underwent SAVR with porcine valve (porcine group) and 410 patients with pericardial valve (pericardial group).
Bioprosthetic valves
Pericardial valves included in the analysis were Carpentier-Edwards Perimount and Perimount Magna (Edwards Lifesciences, Irvine, CA, USA), Sorin Mitroflow and Soprano (LivaNova, London, UK) and St. Jude Trifecta (Abbott, Chicago, IL, USA). Porcine valves included were St. Jude Biocor and Epic Supra (Abbott), Medtronic Hancock II, Hancock II Ultra and Mosaic (Medtronic, Minneapolis, MN, USA). Carpentier-Edwards Perimount Magna (76.8%) and Medtronic Hancock II (56.6%) were the mostly commonly used bioprostheses in the Pericardial and the Porcine groups, respectively. Details on the bioprosthetic valves used are summarized in
Supplementary Table 1.
Data collection
All patients were evaluated with transthoracic doppler echocardiography within 3 months before the index surgery. Postoperative follow-up echocardiographic exams were performed before discharge (3–7 days postoperatively), at 6 to 12 months postoperatively, and then followed annually or biennially. Clinical follow-up information was obtained through visit to the outpatient clinic every 3–6 months.
The primary endpoint was all-cause mortality and structural valve deterioration (SVD), the latter being defined as the structural deterioration of the prostheses requiring for reoperation or that demonstrates severe dysfunction on follow-up echocardiography.
13) Early mortality was defined as those occurring postoperatively within 30 days or in-hospital. The data were collected retrospectively through institutional electronic medical chart system. Data on vital status and date of death were obtained from the institutional electronical database and was also validated by national health claims database of the National Health Insurance Service, a mandatory universal health insurance program providing comprehensive medical care coverage to all residents of the Republic of Korea not to miss inter-visit mortality.
14) The other outcomes of interest were valve-related complication such as thromboembolic events (i.e., stroke or transient ischemic attack), operated valve endocarditis and anticoagulation-related hemorrhage that were defined according to the “Guidelines for Reporting Mortality and Morbidity after Cardiac Valve Interventions” of the Society for the Thoracic Surgeons.
13)
Statistics
Categorical variables, presented as frequencies and percentages, were compared by the use of the χ
2 test or Fisher exact test. Continuous variables, expressed as mean ± SD or median with range, were compared by use of the Student unpaired t-test or the Mann-Whitney U test as appropriate. Kaplan-Meier curves were constructed to delineate overall survival and freedom from adverse valve-related events, and Log-rank test was conducted to test statistical significances in the intergroup differences in the estimates. Logistic regression and Cox proportional hazard model were used to evaluate comparative outcomes in early mortality and long-term outcomes, respectively. In time-to-event analyses, the proportional hazards assumption was tested by examination of log (−log[survival]) curves and by testing of partial (Schoenfeld) residuals and there was a violation in survival outcomes across 2 groups (p=0.003) with a cross over at 8 years. To address this issue, landmark mortality analyses were performed split into 2 intervals: 8 years less and more than 8 years following surgery, in which no relevant violations were found in this landmark model.
15)
To reduce the impact of selection bias in this observational study, we used inverse probability treatment weighting (IPTW) to adjust for all measurable baseline variables. For this, we constructed a propensity score (PS) model, in which the PS is defined as the probability of receiving pericardial valve replacement of individual patients in either of the 2 groups conditional on observed baseline profiles. The PSs were estimated by the logistic regression model including the all 29 baseline variables in
Table 1. Regarding the IPTW modeling, the weight of a patient receiving a pericardial valve was the inverse of the PS, whereas the weight of a patient receiving a porcine valve was the inverse of ‘1 minus PS.’ The maximal level of weighting allowed was 10, and the stabilized-weight was finally given—the weight divided by average weight within the group. After the IPTW, the balance for all baseline covariates was assessed using the standardized mean differences (SMD) and SMD <0.1 was regarded as well-balanced for each variable. Weighted logistic regression and weighted Cox proportional hazard models were used to evaluate the group impacts on early mortality and long-term outcomes, respectively with the adoption of robust standard errors. Postoperative echocardiographic parameters were compared by the use of weighted t-test. For the validation of the results from IPTW, we also performed PS matching. The PS-matched cohorts were generated by matching between the 2 groups on the logit of the PS with the nearest matching algorithm allowing a maximal width of caliper of 0.2 of the SD of the logit of the PS.
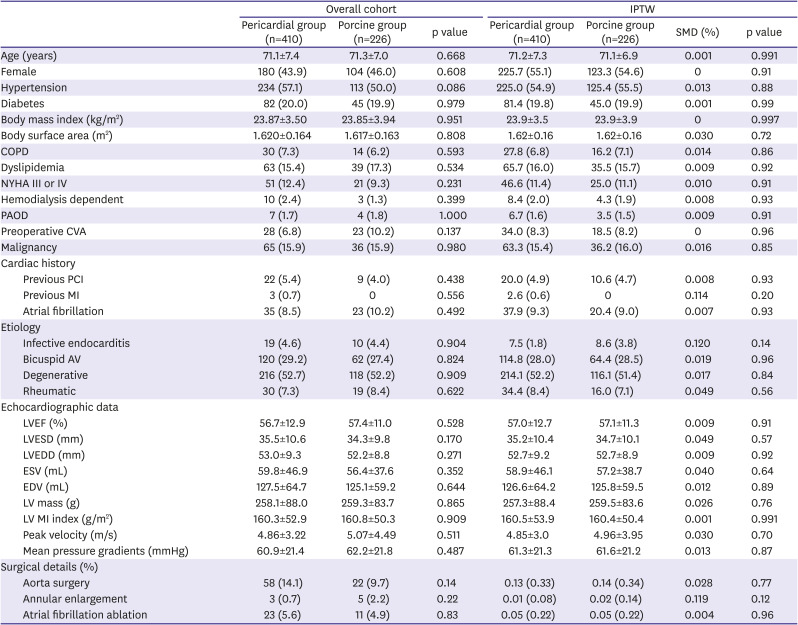
Table 1
Baseline characteristics
|
Overall cohort |
IPTW |
|
Pericardial group (n=410) |
Porcine group (n=226) |
p value |
Pericardial group (n=410) |
Porcine group (n=226) |
SMD (%) |
p value |
|
Age (years) |
71.1±7.4 |
71.3±7.0 |
0.668 |
71.2±7.3 |
71.1±6.9 |
0.001 |
0.991 |
|
Female |
180 (43.9) |
104 (46.0) |
0.608 |
225.7 (55.1) |
123.3 (54.6) |
0 |
0.91 |
|
Hypertension |
234 (57.1) |
113 (50.0) |
0.086 |
225.0 (54.9) |
125.4 (55.5) |
0.013 |
0.88 |
|
Diabetes |
82 (20.0) |
45 (19.9) |
0.979 |
81.4 (19.8) |
45.0 (19.9) |
0.001 |
0.99 |
|
Body mass index (kg/m2) |
23.87±3.50 |
23.85±3.94 |
0.951 |
23.9±3.5 |
23.9±3.9 |
0 |
0.997 |
|
Body surface area (m2) |
1.620±0.164 |
1.617±0.163 |
0.808 |
1.62±0.16 |
1.62±0.16 |
0.030 |
0.72 |
|
COPD |
30 (7.3) |
14 (6.2) |
0.593 |
27.8 (6.8) |
16.2 (7.1) |
0.014 |
0.86 |
|
Dyslipidemia |
63 (15.4) |
39 (17.3) |
0.534 |
65.7 (16.0) |
35.5 (15.7) |
0.009 |
0.92 |
|
NYHA III or IV |
51 (12.4) |
21 (9.3) |
0.231 |
46.6 (11.4) |
25.0 (11.1) |
0.010 |
0.91 |
|
Hemodialysis dependent |
10 (2.4) |
3 (1.3) |
0.399 |
8.4 (2.0) |
4.3 (1.9) |
0.008 |
0.93 |
|
PAOD |
7 (1.7) |
4 (1.8) |
1.000 |
6.7 (1.6) |
3.5 (1.5) |
0.009 |
0.91 |
|
Preoperative CVA |
28 (6.8) |
23 (10.2) |
0.137 |
34.0 (8.3) |
18.5 (8.2) |
0 |
0.96 |
|
Malignancy |
65 (15.9) |
36 (15.9) |
0.980 |
63.3 (15.4) |
36.2 (16.0) |
0.016 |
0.85 |
|
Cardiac history |
|
|
|
|
|
|
|
|
Previous PCI |
22 (5.4) |
9 (4.0) |
0.438 |
20.0 (4.9) |
10.6 (4.7) |
0.008 |
0.93 |
|
Previous MI |
3 (0.7) |
0 |
0.556 |
2.6 (0.6) |
0 |
0.114 |
0.20 |
|
Atrial fibrillation |
35 (8.5) |
23 (10.2) |
0.492 |
37.9 (9.3) |
20.4 (9.0) |
0.007 |
0.93 |
|
Etiology |
|
|
|
|
|
|
|
|
Infective endocarditis |
19 (4.6) |
10 (4.4) |
0.904 |
7.5 (1.8) |
8.6 (3.8) |
0.120 |
0.14 |
|
Bicuspid AV |
120 (29.2) |
62 (27.4) |
0.824 |
114.8 (28.0) |
64.4 (28.5) |
0.019 |
0.96 |
|
Degenerative |
216 (52.7) |
118 (52.2) |
0.909 |
214.1 (52.2) |
116.1 (51.4) |
0.017 |
0.84 |
|
Rheumatic |
30 (7.3) |
19 (8.4) |
0.622 |
34.4 (8.4) |
16.0 (7.1) |
0.049 |
0.56 |
|
Echocardiographic data |
|
|
|
|
|
|
|
|
LVEF (%) |
56.7±12.9 |
57.4±11.0 |
0.528 |
57.0±12.7 |
57.1±11.3 |
0.009 |
0.91 |
|
LVESD (mm) |
35.5±10.6 |
34.3±9.8 |
0.170 |
35.2±10.4 |
34.7±10.1 |
0.049 |
0.57 |
|
LVEDD (mm) |
53.0±9.3 |
52.2±8.8 |
0.271 |
52.7±9.2 |
52.7±8.9 |
0.009 |
0.92 |
|
ESV (mL) |
59.8±46.9 |
56.4±37.6 |
0.352 |
58.9±46.1 |
57.2±38.7 |
0.040 |
0.64 |
|
EDV (mL) |
127.5±64.7 |
125.1±59.2 |
0.644 |
126.6±64.2 |
125.8±59.5 |
0.012 |
0.89 |
|
LV mass (g) |
258.1±88.0 |
259.3±83.7 |
0.865 |
257.3±88.4 |
259.5±83.6 |
0.026 |
0.76 |
|
LV MI index (g/m2) |
160.3±52.9 |
160.8±50.3 |
0.909 |
160.5±53.9 |
160.4±50.4 |
0.001 |
0.991 |
|
Peak velocity (m/s) |
4.86±3.22 |
5.07±4.49 |
0.511 |
4.85±3.0 |
4.96±3.95 |
0.030 |
0.70 |
|
Mean pressure gradients (mmHg) |
60.9±21.4 |
62.2±21.8 |
0.487 |
61.3±21.3 |
61.6±21.2 |
0.013 |
0.87 |
|
Surgical details (%) |
|
|
|
|
|
|
|
|
Aorta surgery |
58 (14.1) |
22 (9.7) |
0.14 |
0.13 (0.33) |
0.14 (0.34) |
0.028 |
0.77 |
|
Annular enlargement |
3 (0.7) |
5 (2.2) |
0.22 |
0.01 (0.08) |
0.02 (0.14) |
0.119 |
0.12 |
|
Atrial fibrillation ablation |
23 (5.6) |
11 (4.9) |
0.83 |
0.05 (0.22) |
0.05 (0.22) |
0.004 |
0.96 |

All reported p values were 2-sided and the p values of <0.05 were considered statistically significant. R software version 3.4.0 (R Foundation, Vienna, Austria;
http://www.R-project.org/) was used for statistical analyses.
RESULTS
Crude analyses
Baseline characteristics are summarized in
Table 1. There were no overt discriminating baseline variables between the 2 groups including demographic characteristics, clinical risk profiles, echocardiographic measures and combined surgical procedures. Early mortality occurred in 20 (3.1%) patients without a significant inter-group difference (p=0.81). The mean follow-up duration was 60.1±50.2 months (63.5±48.7 months for the porcine group and 58.2±51.0 months for the pericardial group). Late mortality occurred in 226 patients (7.8%/patient-year [PY]). Unadjusted overall survival rates were not significantly different between 2 groups: 5- and 10-year survival rates were 83.1% and 48.0%, respectively in the pericardial group, and were 75.6% and 50.1%, respectively in the porcine group (p=0.80;
Figure 1A). SVD occurred in 11 patients—6 in the pericardial and 5 in the porcine groups, details of which are presented in
Supplementary Table 2. Overall all 8 out of the 11 patients with SVD underwent reintervention either by SAVR or TAVR. Among the 3 patients with SVD who did not undergo reintervention, 2 clinically stable patients have been on follow-up without reintervention while the other symptomatic patient was recommended to undergo surgery with resultant refusal from the patient. Overall, there was no significance difference in the rates of SVD between the groups (
Figure 1B).
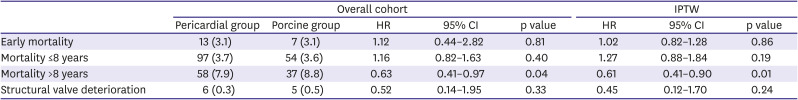
Table 2 summarizes the comparative outcomes for primary endpoints. In addition, permanent pacemaker was implanted in 10 patients (2.4%) in pericardial group and 2 patients (0.8%) in the porcine group (p=0.17).
Figure 1
(A) Unadjusted overall survival rates between pericardial and porcine groups. The 5- and 10-year survival rates were 82.2% and 56.3%, respectively in the pericardial group, and were 86.5% and 64.9%, respectively in the porcine group. (B) Unadjusted freedom from SVD between pericardial and porcine groups.
SVD = structural valve deterioration.
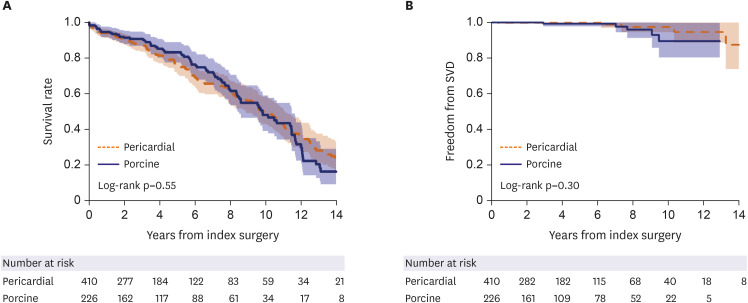

Table 2
Crude and adjusted primary study outcomes
|
Overall cohort |
IPTW |
|
Pericardial group |
Porcine group |
HR |
95% CI |
p value |
HR |
95% CI |
p value |
|
Early mortality |
13 (3.1) |
7 (3.1) |
1.12 |
0.44–2.82 |
0.81 |
1.02 |
0.82–1.28 |
0.86 |
|
Mortality ≤8 years |
97 (3.7) |
54 (3.6) |
1.16 |
0.82–1.63 |
0.40 |
1.27 |
0.88–1.84 |
0.19 |
|
Mortality >8 years |
58 (7.9) |
37 (8.8) |
0.63 |
0.41–0.97 |
0.04 |
0.61 |
0.41–0.90 |
0.01 |
|
Structural valve deterioration |
6 (0.3) |
5 (0.5) |
0.52 |
0.14–1.95 |
0.33 |
0.45 |
0.12–1.70 |
0.24 |

Details on other valve-related adverse outcomes are summarized in
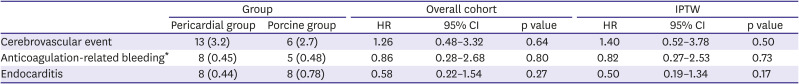
Table 3. Cerebrovascular events occurred in 19 patients (6 in the porcine group, 13 in the pericardial group). Of these, 5 patients had cerebral hemorrhage and 14 patients had ischemic stroke. There were no significant differences in the incidences in stroke, anticoagulation-related bleeding and endocarditis between groups.
Table 3
Major other valve-related events
|
Group |
Overall cohort |
IPTW |
|
Pericardial group |
Porcine group |
HR |
95% CI |
p value |
HR |
95% CI |
p value |
|
Cerebrovascular event |
13 (3.2) |
6 (2.7) |
1.26 |
0.48–3.32 |
0.64 |
1.40 |
0.52–3.78 |
0.50 |
|
Anticoagulation-related bleeding*
|
8 (0.45) |
5 (0.48) |
0.86 |
0.28–2.68 |
0.80 |
0.82 |
0.27–2.53 |
0.73 |
|
Endocarditis |
8 (0.44) |
8 (0.78) |
0.58 |
0.22–1.54 |
0.27 |
0.50 |
0.19–1.34 |
0.17 |

Adjusted analyses
PS model yielded C-statistics of 0.60 (95% confidence interval [CI], 0.55–0.65) and the distributions of the PS between the 2 groups before and after IPTW are illustrated in
Supplementary Figure 1. By the adjustment using IPTW modeling, all the baseline covariates remained similar across the 2 groups (
Table 1, right-side columns). After adjustment, risks of early mortality and SVD were comparable for both groups (
Figure 2;
Table 2, right-side columns) as well as for other valve-related outcomes (
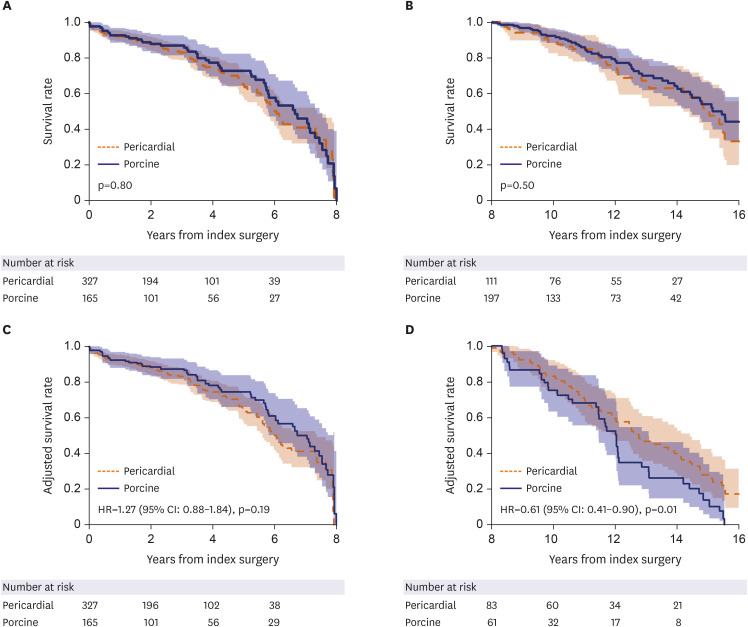
Table 3, right-side columns). The landmark survival analyses showed comparable survival rates between the 2 groups within 8 years following surgery (hazard ratio [HR], 1.27; 95% CI, 0.88–1.84; p=0.19), however, the pericardial group showed a significantly superior survival trend beyond 8 years after surgery as compared with the porcine group (HR, 0.61; 95% CI, 0.41–0.90; p=0.010;
Figure 3).
Figure 2
Adjusted freedom from structural valve deterioration using inverse probability treatment weighting modeling between pericardial and porcine groups.
SVD = structural valve deterioration; HR = hazard ratio; CI = confidence interval.
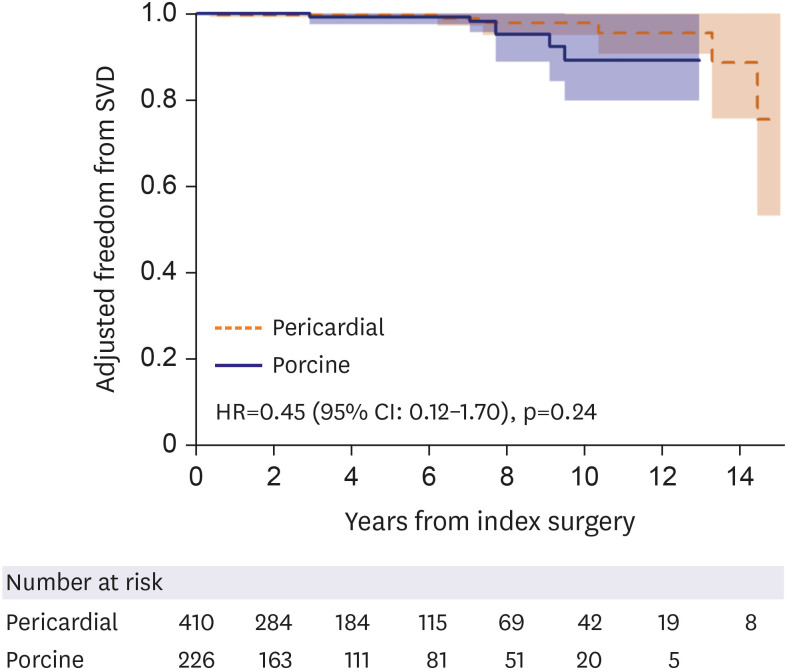

Figure 3
Landmark survival plots for unadjusted groups (A, B) and adjusted groups by inverse-probability treatment weighting (C, D) across 8 years following surgery between the pericardial and the porcine groups: (A) crude within 8 years, (B) crude beyond 8 years, (C) adjusted within 8 years and (D) adjusted beyond 8 years following surgery.

Hemodynamic performance
A total of 1,141 echocardiographic records were available in 636 patients including post-discharge echocardiography in 632 patients (99.4%). While preoperative mean pressure gradients across AV were similar between groups, postoperative transthoracic echocardiography demonstrated lower mean pressure gradient in the pericardial group over porcine group done at both immediate postoperative point (at median 4 postoperative days; quartile 1–3, 4–6 days) and latest follow-up examination (at median 28 months; quartile 1–3, 12–62 months) with a trendy of widening in the inter-group discrepancy over time (
Table 4). These results remained constant even after adjustment with IPTW (
Table 4, right-side columns;
Figure 4).
Figure 4
Changes in mean pressure gradients across the aortic valve over time (preoperative, early postoperative, last follow-up) in pericardial group (A) and porcine group (B).
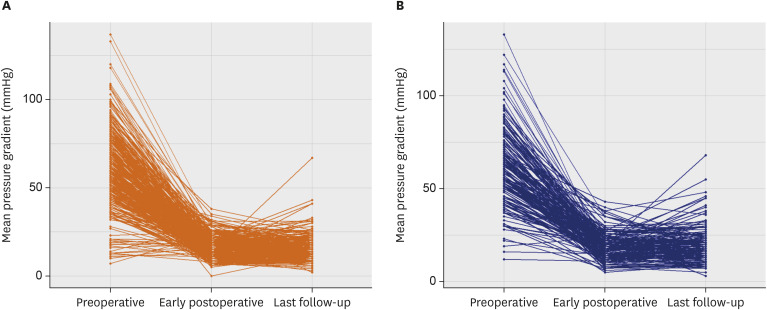

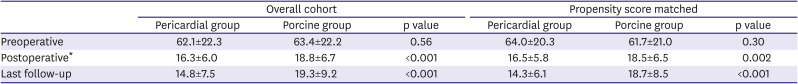
Table 4
Change of transvalvular mean pressure gradient
|
Overall cohort |
Propensity score matched |
|
Pericardial group |
Porcine group |
p value |
Pericardial group |
Porcine group |
p value |
|
Preoperative |
62.1±22.3 |
63.4±22.2 |
0.56 |
64.0±20.3 |
61.7±21.0 |
0.30 |
|
Postoperative*
|
16.3±6.0 |
18.8±6.7 |
<0.001 |
16.5±5.8 |
18.5±6.5 |
0.002 |
|
Last follow-up |
14.8±7.5 |
19.3±9.2 |
<0.001 |
14.3±6.1 |
18.7±8.5 |
<0.001 |

We also compared pre- and postoperative left ventricular (LV) mass between the 2 groups, in which there was no significant inter-group difference in the level of LV mass on latest follow-up echocardiographic evaluation (p=0.73;
Supplementary Figure 2).
DISCUSSION
Despite the increasing uses of bioprostheses over mechanical valve in the heart valve replacement surgeries in recent years, their principal limitation is the development of SVD over time with resultant needs for re-intervention. In the present study, the transvalvular mean pressure gradient was significantly lower in the pericardial group than the porcine group in immediate postoperative period (16.3±6.0 mmHg vs. 18.8±6.7 mmHg, p<0.001) and the effect was consistent during the late follow-up period. Although the superior hemodynamic profiles of bovine pericardial valves could not be translated into significantly superior durability than porcine valves in the present study, there have been reports that high transvalvular mean pressure gradient is associated with development of SVD. In a large observational study involving 12,569 patients who underwent AVR using bovine pericardial valve (Carpentier-Edwards Perimount; Edwards Lifesciences), Johnston et al.
16) reported that higher transvalvular pressure gradient, prosthesis-patient mismatch and younger age lesser than 60 years were associated with higher risks of reintervention for SVD during median follow-up period of 5.8 years. Furthermore, Gao et al.
12) showed that 10-year freedom from valve-explant was superior in the pericardial group than the porcine group (97±1% vs. 90±2%, p=0.04).
Meanwhile, Dalmau et al.
17) randomized 108 patients undergoing AVR to receive either pericardial valve (Carpentier-Edwards Perimount Magna; Edwards Lifesciences) or porcine valve (Medtronic Mosaic; Medtronic). They reported survival at 5 years to be 94.4% in pericardial group and 79.6% in porcine group (p=0.039) and postulated that this difference might be attributable to favorable hemodynamics with its consequent improved LV mass regression in the pericardial valve replacement group. On the other hand, there have been studies consistently reporting comparable long-term survival between pericardial and valves despite the better hemodynamics in the pericardial valves.
5)8)10)11)12)18)19) Such mixed results in the prior studies might have been attributable to relatively small sample sizes of each studies, heterogeneity in the subject populations and inclusion of only selected pericardial and porcine valves, and they also have a limitation of not being able to evaluate on more recent valves that are now in active uses in the real world. Consequently, decision-making for selecting ideal prostheses for SAVR among all available bioprosthetic valves has been challenged to date.
During the follow-up period of 60.1±50.2 months in the present study, freedom from SVD was not statistically different between groups while it was numerically better in the pericardial group than the porcine group (incidence rates: 0.31%/PY vs. 0.50%/PY; HR, 0.45). The statistical insignificant may stem from a small number of SVDs (n=11) to generate sufficient statistical power, and therefore, this needs to be addressed by studies on larger cohort with longer follow-ups. Nonetheless, survival outcomes turned out to be superior (HR, 0.61; 95% CI, 0.41–0.90; p=0.01) more than 8 years after surgery favoring pericardial valves. These survival difference in the late period (>8 years) may be attributable either to superior hemodynamic profiles or to better durability of bovine pericardial valves over porcine valves, in which the mode of valve failure in porcine valve is mainly acute cusp tears, whereas bovine valves tend to fail more gradually with increasing stenosis. We, however, could not demonstrate the cause of the significant survival difference in the late period from the present dataset. Of note, there were no significant intergroup differences in the rate of SVD and valve-reoperation between the groups even after statistical adjustments, however, given perceived high barrier to undertake valve-reoperation in elderly patients with multiple comorbidity that are usual pictures long after valve replacement surgery, the rate of reoperation may not be a fair surrogate of valve durability in bioprosthetic valve replacement. Further studies with targeted investigations on the modes of bioprosthetic valve failure, ideally in forms of prospective manner, would be helpful to validate our results.
The current study has several limitations inherent to a retrospective analysis of observational data from a single center. Although we did use a rigorous statistical adjustment methods, in the absence of randomization, selection bias or detection bias may have affected the results and thus the conclusions. Additionally, this study included various bioprosthetic valves and this could increase heterogeneity. Finally, relatively small numbers of each clinical events, SVD in particular, may limit statistical power to offer robust comparability between the groups.
In patients undergoing SAVR, the use of bovine pericardial valves was associated with superior prosthetic AV hemodynamic profiles and improved late survival as compared with porcine valves. Freedom from adverse valve-related complication rates were not significantly different between the 2 groups.












 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download