1. Waxman SG. Control of movement. Waxman SG, editor. Clinical Neuroanatomy. 28th ed. New York, NY, USA: McGraw-Hill Education;2017.
2. Hänggi J, Langer N, Lutz K, Birrer K, Mérillat S, Jäncke L. Structural brain correlates associated with professional handball playing. PLoS One. 2015; 10(4):e0124222. PMID:
25915906.
3. Park IS, Han JW, Lee KJ, Lee NJ, Lee WT, Park KA, et al. Evaluation of morphological plasticity in the cerebella of basketball players with MRI. J Korean Med Sci. 2006; 21(2):342–346. PMID:
16614526.
4. Park IS, Lee KJ, Han JW, Lee NJ, Lee WT, Park KA, et al. Experience-dependent plasticity of cerebellar vermis in basketball players. Cerebellum. 2009; 8(3):334–339. PMID:
19259755.
5. Park IS, Lee YN, Kwon S, Lee NJ, Rhyu IJ. White matter plasticity in the cerebellum of elite basketball athletes. Anat Cell Biol. 2015; 48(4):262–267. PMID:
26770877.
6. Park IS, Lee KJ, Han JW, Lee NJ, Lee WT, Park KA, et al. Basketball training increases striatum volume. Hum Mov Sci. 2011; 30(1):56–62. PMID:
21030099.
7. Park IS, Lee NJ, Rhyu IJ. Roles of the declive, folium, and tuber cerebellar vermian lobules in sportspeople. J Clin Neurol. 2018; 14(1):1–7. PMID:
29141275.
8. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007; 38(1):95–113. PMID:
17761438.
9. Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1994; 2(4):189–210.
10. Duvernov HM. The Human Brain: Surface, Three-Dimensional Sectional Anatomy, and MRI. New York, NY, USA: Springer-Verlag;1999.
11. Dahnke R, Yotter RA, Gaser C. Cortical thickness and central surface estimation. Neuroimage. 2013; 65:336–348. PMID:
23041529.
12. Yotter RA, Nenadic I, Ziegler G, Thompson PM, Gaser C. Local cortical surface complexity maps from spherical harmonic reconstructions. Neuroimage. 2011; 56(3):961–973. PMID:
21315159.
13. Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010; 53(1):1–15. PMID:
20547229.
14. Vanderah TW, Gould DJ. Nolte’s the Human Brain: An Introduction to Its Functional Anatomy. 8th ed. Philadelphia, PA, USA: Elsevier;2021.
15. Kumar N, Manning TF, Ostry DJ. Somatosensory cortex participates in the consolidation of human motor memory. PLoS Biol. 2019; 17(10):e3000469. PMID:
31613874.
16. Jacini WF, Cannonieri GC, Fernandes PT, Bonilha L, Cendes F, Li LM. Can exercise shape your brain? Cortical differences associated with judo practice. J Sci Med Sport. 2009; 12(6):688–690. PMID:
19147406.
17. Huang R, Lu M, Song Z, Wang J. Long-term intensive training induced brain structural changes in world class gymnasts. Brain Struct Funct. 2015; 220(2):625–644. PMID:
24297657.
18. Karnath HO, Ferber S, Himmelbach M. Spatial awareness is a function of the temporal not the posterior parietal lobe. Nature. 2001; 411(6840):950–953. PMID:
11418859.
19. Tracy J, Flanders A, Madi S, Laskas J, Stoddard E, Pyrros A, et al. Regional brain activation associated with different performance patterns during learning of a complex motor skill. Cereb Cortex. 2003; 13(9):904–910. PMID:
12902389.
20. Hall NM, Gjedde A, Kupers R. Neural mechanisms of voluntary and involuntary recall: a PET study. Behav Brain Res. 2008; 186(2):261–272. PMID:
17913256.
21. Barrett AM, Abdou A, Caulfield MD. The cingulate cortex and spatial neglect. Handb Clin Neurol. 2019; 166:129–150. PMID:
31731909.
22. Auger SD, Maguire EA. Assessing the mechanism of response in the retrosplenial cortex of good and poor navigators. Cortex. 2013; 49(10):2904–2913. PMID:
24012136.
23. Im K, Lee JM, Yoon U, Shin YW, Hong SB, Kim IY, et al. Fractal dimension in human cortical surface: multiple regression analysis with cortical thickness, sulcal depth, and folding area. Hum Brain Mapp. 2006; 27(12):994–1003. PMID:
16671080.
24. Blanton RE, Levitt JG, Thompson PM, Narr KL, Capetillo-Cunliffe L, Nobel A, et al. Mapping cortical asymmetry and complexity patterns in normal children. Psychiatry Res. 2001; 107(1):29–43. PMID:
11472862.
25. Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci U S A. 1990; 87(14):5568–5572. PMID:
1695380.
26. Kim HT, Kim IH, Lee KJ, Lee JR, Park SK, Chun YH, et al. Specific plasticity of parallel fiber/Purkinje cell spine synapses by motor skill learning. Neuroreport. 2002; 13(13):1607–1610. PMID:
12352611.
27. Lee KJ, Jung JG, Arii T, Imoto K, Rhyu IJ. Morphological changes in dendritic spines of Purkinje cells associated with motor learning. Neurobiol Learn Mem. 2007; 88(4):445–450. PMID:
17720555.
28. Lee KJ, Park IS, Kim H, Greenough WT, Pak DT, Rhyu IJ. Motor skill training induces coordinated strengthening and weakening between neighboring synapses. J Neurosci. 2013; 33(23):9794–9799. PMID:
23739975.
29. Kim YH, Park JW, Ko MH, Jang SH, Lee PK. Plastic changes of motor network after constraint-induced movement therapy. Yonsei Med J. 2004; 45(2):241–246. PMID:
15118995.
30. Lee KJ, Rhyu IJ. Effects of exercise on structural and functional changes in the aging brain. J Korean Med Assoc. 2009; 52(9):907–919.
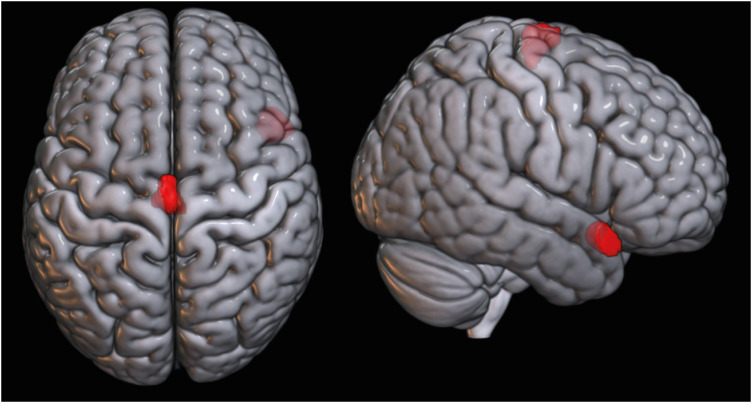
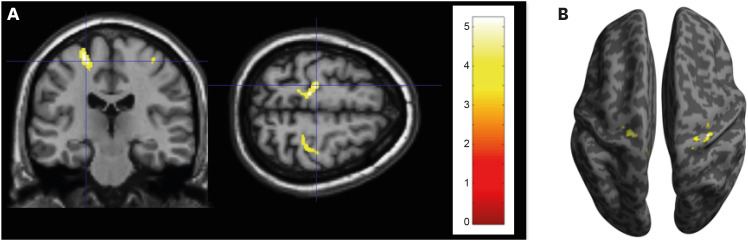




 PDF
PDF Citation
Citation Print
Print



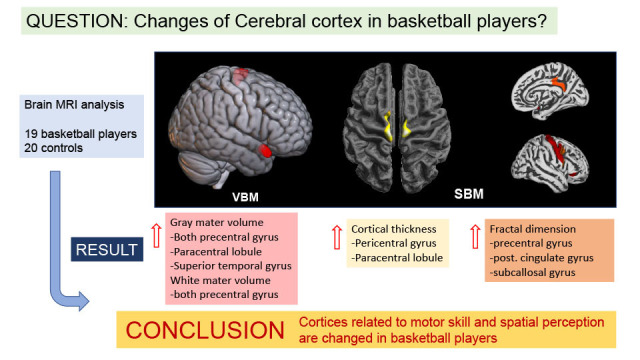
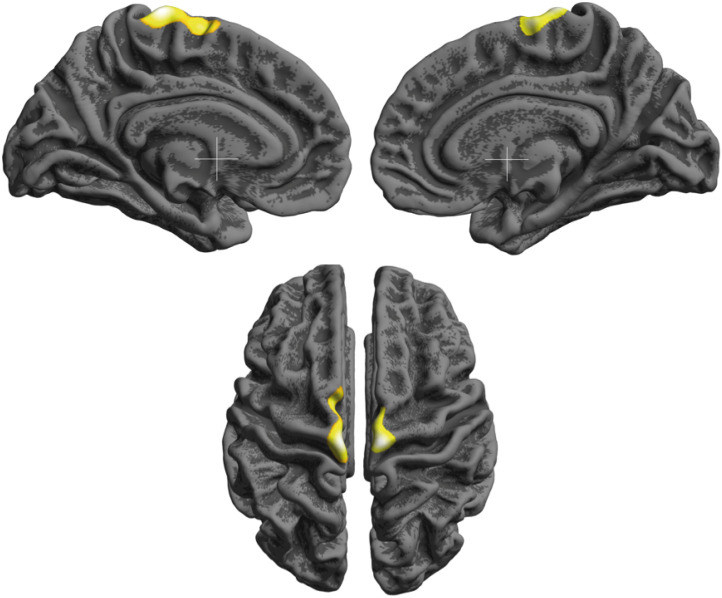
 XML Download
XML Download