Introduction
Peri-implantitis refers to irreversible inflammation of the tissues surrounding a dental implant and can result in implant loss if left untreated.
1 A recent systematic review reported that the prevalence of peri-implant mucositis, which is the inflammation of the soft tissue surrounding a dental implant, is 43% while that of peri-implantitis, which is the inflammation of the soft and hard tissues surrounding a dental implant, is 22%.
2 Since the mid-1960s, implants have become an established treatment method for replacement of missing teeth. It is important to implement measures against implant-related complications since the incidence of these complications continues to rise.
3
Peri-implantitis treatment methods are largely categorized as non-surgical or surgical method. Non-surgical methods are essential in preparing tissues before the surgical procedure and include the use of curettes of various shapes to clean the implant surface, prescription of local or systemic antibiotics, or removal of the bacterial biofilm by laser or ultrasound.
4 Non-surgical methods provide limited accessibility to the implant compared to surgical methods and do not allow simultaneous regeneration of hard and soft tissues; thus, they have limited efficacy in the management of peri-implantitis accompanied by bone loss.
5 Owing to these limitations, surgical management is deemed necessary.
Surgical methods offer high implant accessibility and wide visualization during treatment of peri-implantitis accompanied by tissue loss, and are classified as access flap, resective, or regenerative approaches. The choice between these options is based on the shape of the bone defect. Resective procedures are recommended for horizontal bone defects, while regenerative procedures are recommended for vertical bone defects; a combination of the two is recommended for complex bone defects.
6
Studies have reported decontamination of the implant surface with hydrogen peroxide following flap elevation, Teflon curettes and abrasive sodium carbonate air powder, titanium-coated Gracey curettes or carbon fiber curettes with subsequent systemic antibiotic administration, 0.2% chlorhexidine mouthwash, and placement of a bone graft and collagen membrane over the defect.
7,8 While these methods have achieved clinical improvements and their long-term effects have been assessed, evidence regarding the effectiveness of these methods is still lacking.
9
A rotary titanium brush (R-Brush, NeoBiotech, Seoul, Korea) with titanium bristles was developed to decontaminate and change the surface topology of an implant affected by peri-implantitis. The brush may be useful for Class Ie peri-implant defects characterized by circumferential bone loss with intact buccal and lingual bone or Class II peri-implant defects characterized by horizontal and supra-alveolar bone loss.
10,11 This case report presents three cases of severe peri-implantitis treated by non-surgical methods followed by surgical therapy involving a rotary titanium brush and guided bone regeneration (GBR). The study protocol was approved by the institutional review board (BOHUN IRB No. 2021-01-042).
Go to :

Discussion
A 2019 Congress Scientific Report (28th European Association for Osseointegration) extensively categorizes peri-implant treatment methods into plaque control capacity, reduction of probing depth, implant surface decontamination, and bone reconstruction.
6 To achieve these therapeutic goals, cleaning of the implant is important. Mechanical cleaning of an implant has limited efficacy owing to the shape of the implant screw. One study reported the lowest cleaning efficacy for the periapical sides of screw threads, followed by the areas between threads and sand thread tips.
13 Among Gracey steel curettes, ultrasonic device with a steel tip, and air powder abrasives with glycine powder used to clean implants, air powder abrasives had the highest cleaning efficacy.
13
Mechanical cleaning causes macroscopic and microscopic changes in the surface topography of an implant, thereby altering the surface roughness of the implant. A study examining the effect of five tools (metal scaler, Teflon tip, two types of titanium brush, and glycine air abrasive) on the surface topography of an implant reported that a metal scaler changes the macroscopic topography of an implant surface, whereas a Teflon tip leaves remnants of the plastic tip. While a metal scaler increased the surface roughness between the screw threads, the titanium brush and glycine air abrasives reduced the surface roughness between the screw threads. However, statistically significant results were observed only for titanium brushes, and they can be effectively used to clean implant valleys and reduce implant surface roughness.
14
Implantoplasty may be performed to reduce the surface roughness of an implant to improve treatment outcomes. However, the procedure can increase the risk of fracture of implants with a small diameter,
15 and the titanium fragments resulting from the fracture can affect the surrounding tissues.
16
The shape of a bone defect plays a crucial role on the outcome of a regenerative procedure performed following implant surface cleaning.
17 A study reported that regenerative procedures produce better outcomes for circumferential bone defects with buccal and lingual bone plates than without bone plates.
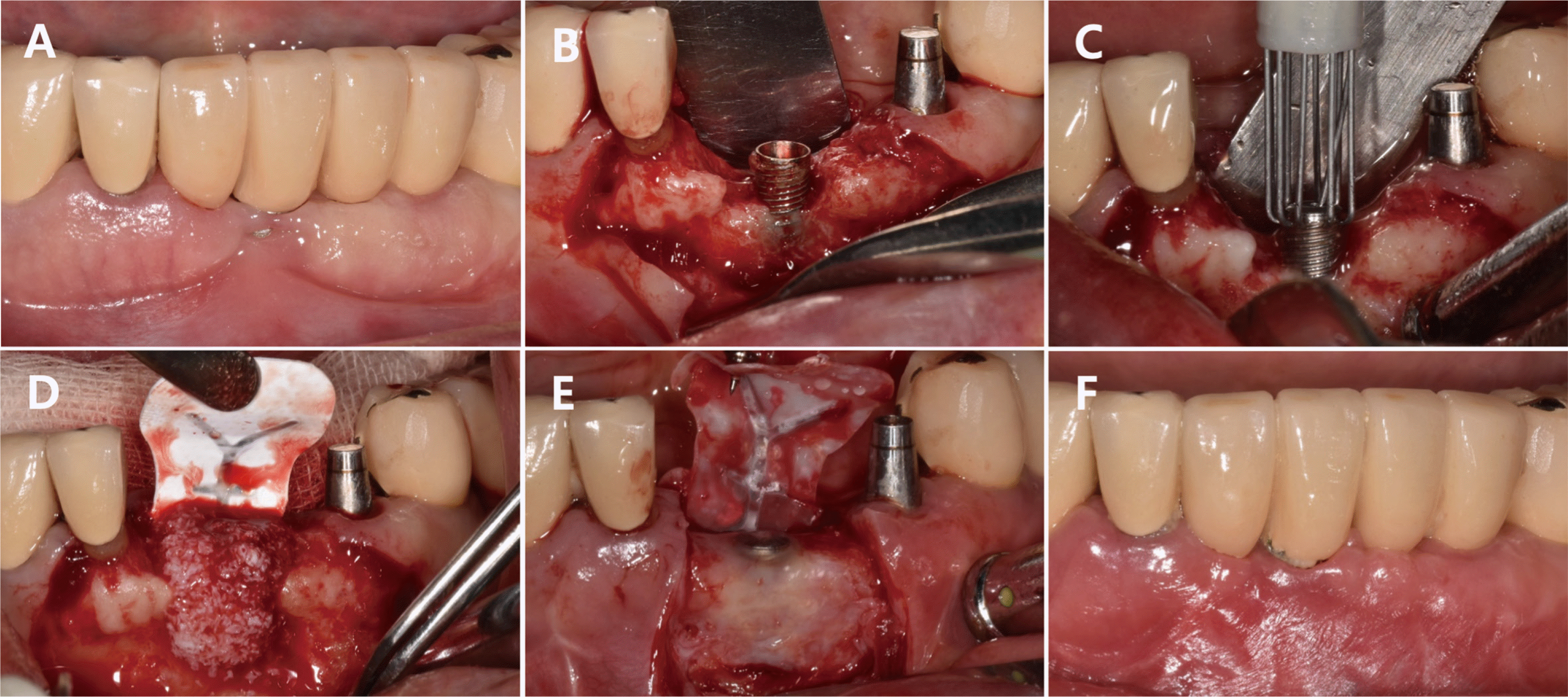
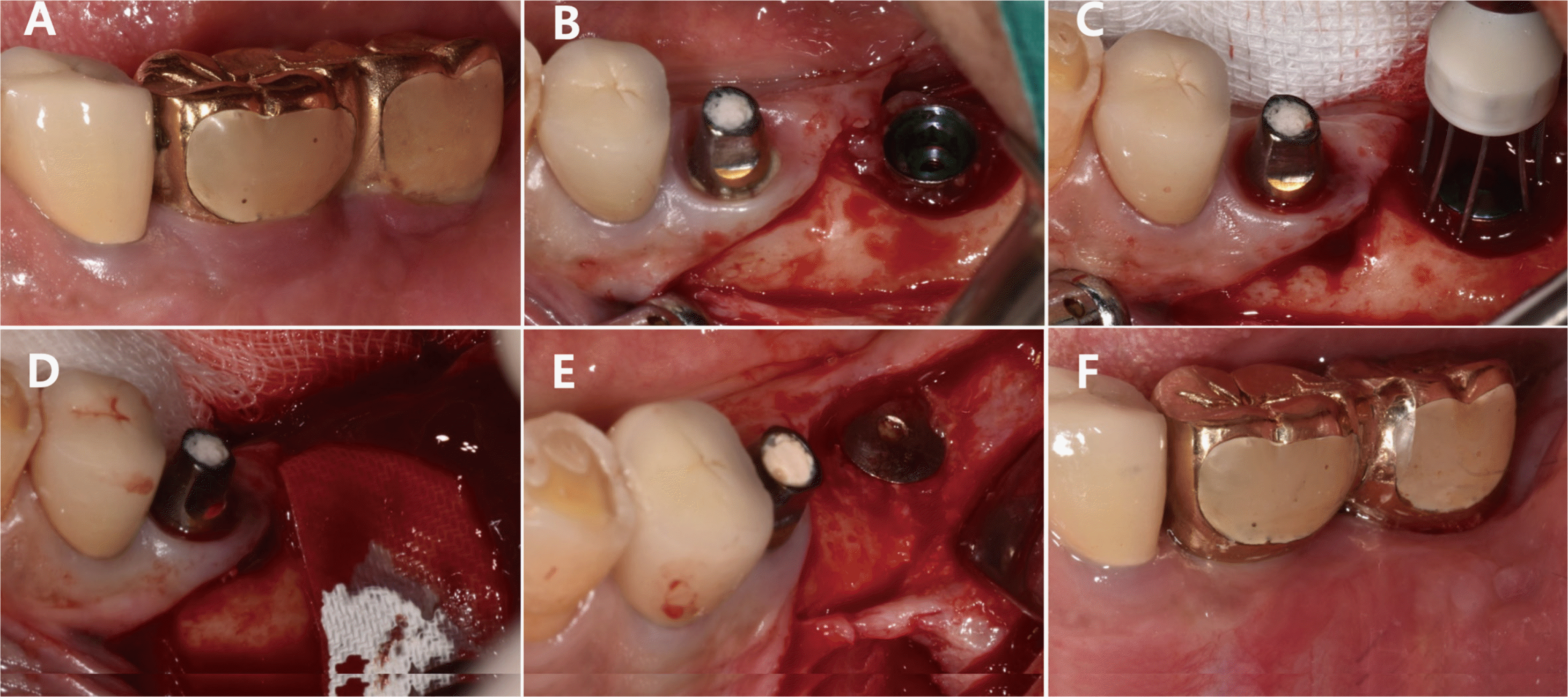
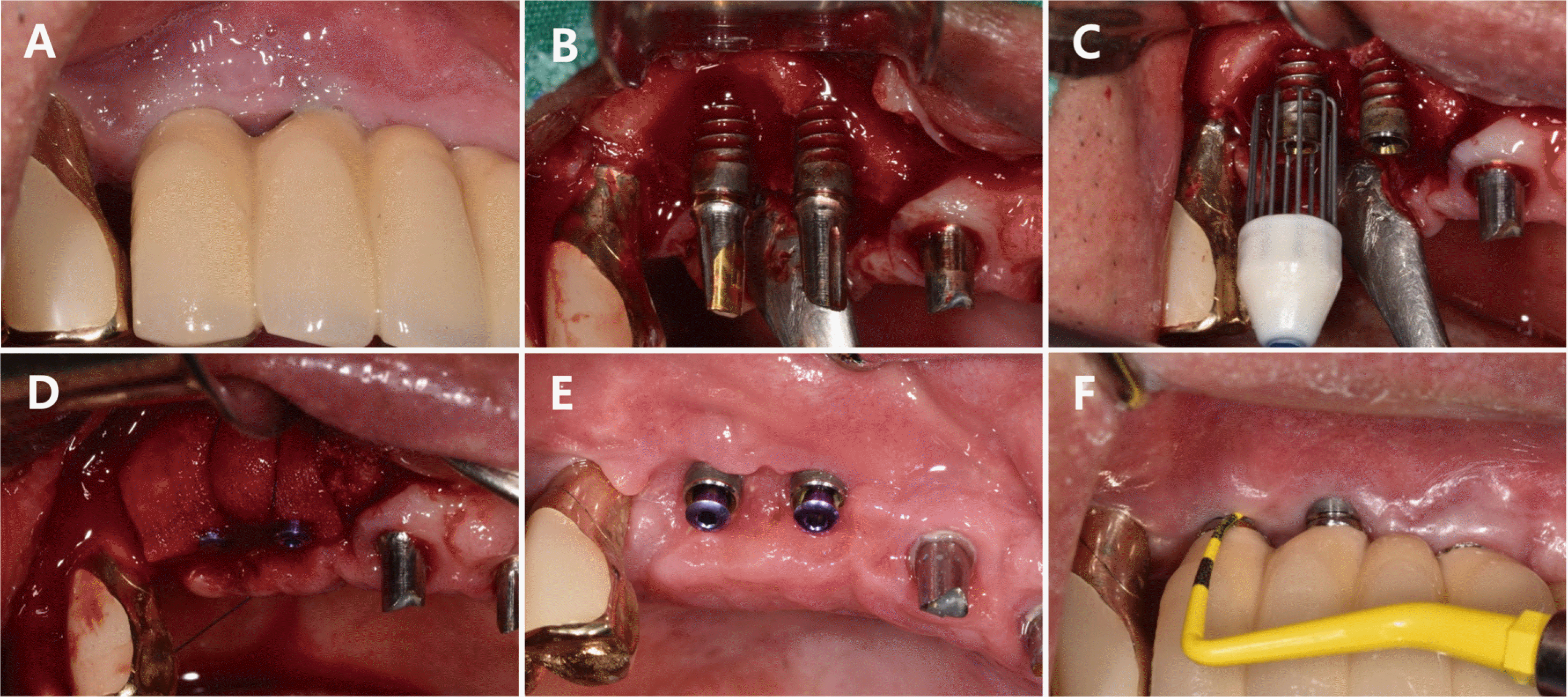
The bone defects in this case report were classified and diagnosed as supra-alveolar, circumferential, and intra-bony defects in Cases 1, 2, and 3, respectively. Although the same bone graft material was used for all three cases, different types of membrane were applied since the shapes of the bone defects varied across the cases. For Case 1, graft placement was deemed difficult since both the buccal and lingual bone plates were lost. As a result, a titanium-reinforced dense polytetrafluoroethylene membrane was placed, followed by a soft tissue graft.
During the re-entry surgery, the sites of bone loss surrounding the implants in Cases 1 and 2 were observed to have healed. Since re-entry surgery for early implant exposure was not performed in Case 3, the healing progress at the site of the bone defect could not be examined in this case. The marginal bone was not restored until above the crestal module for Cases 1 and 3, unlike Case 2, based on radiographic and clinical findings (
Fig. 4). Additionally, Case 3 showed buccal gingival recession and rough surface exposure (
Fig. 3,
Table 1). Postoperative radiographs revealed that the bone graft did not cover the upper portion of the crestal module for Case 1, unlike in Cases 2 and 3. Case 3 failed to achieve primary wound closure, unlike in Cases 1 and 2. The depth of the implant placement and the design of the upper portion of an implant have been reported to affect marginal bone formation and resorption.
18 However, the mean mPI, mSBI, PD, and DIB of the three cases decreased from 1.12 to 0.19, 1.38 to 0.25, 8.73 mm to 3 mm, and 6.35 to 2.3 mm, respectively, over the relatively short follow-up period of two years. The bone fill of approximately 4 mm in this case was comparable to the 1.9 - 4.2 mm bone fill observed in previous studies
7 on GBR. The reduction of probing depth in these cases was good compared to that obtained in other studies using a rotary titanium brush.
11 Improvements in symptoms, such as an absence of discomfort, were also reported.
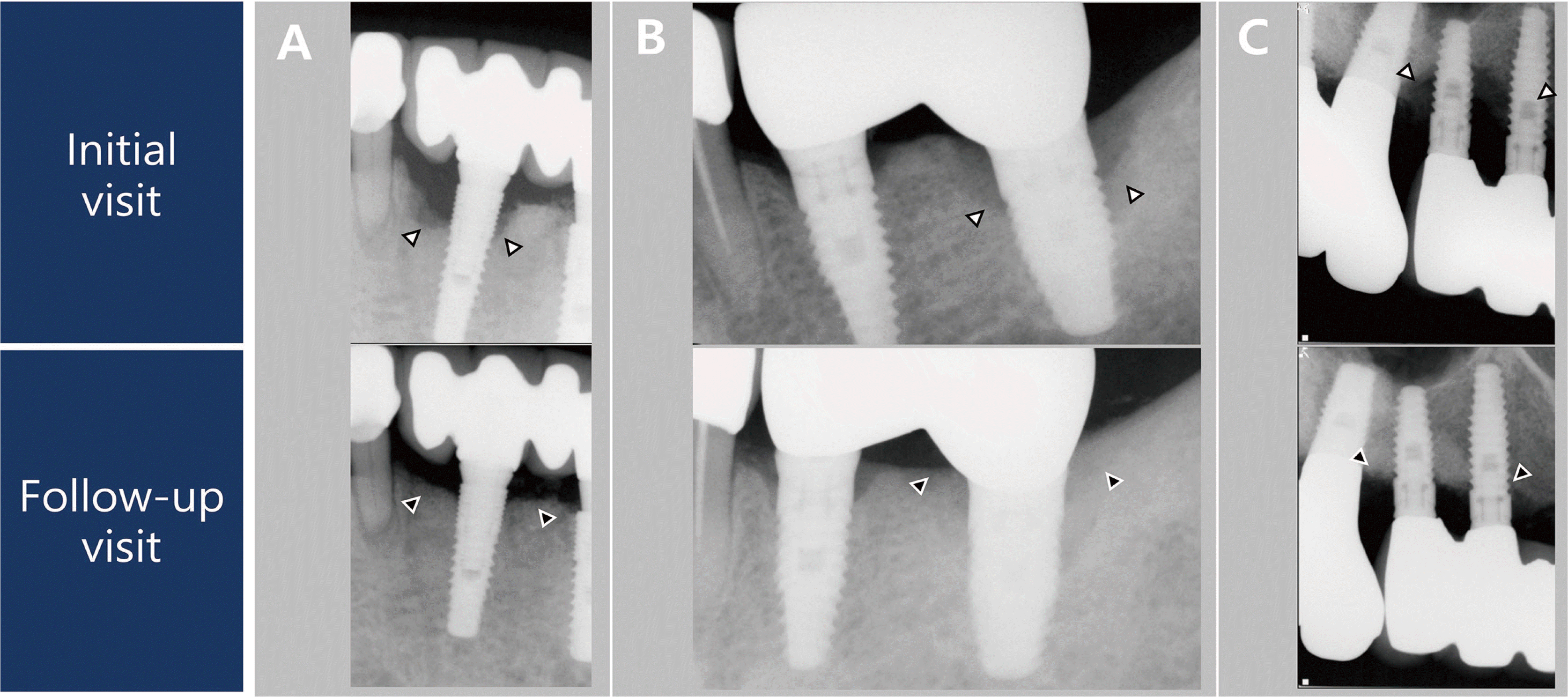 | Fig. 4Intra-oral radiographs on baseline and follow-up visits. (A) Case 1, (B) Case 2, and (C) Case 3. Black and white arrows indicate the marginal bone level on baseline and follow-up visits, respectively. 
|
All three patients required long-term follow-up. The use of a titanium brush may improve the prognosis of a regenerative procedure as it cleans the implant surface and maintains the implant surface roughness and shape of screw threads. However, the risk of implant fragment generation during tool manipulation should be considered. A titanium brush should be used with caution and sufficient irrigation to reduce the risk of implant fragment generation.
19 However, following the regenerative procedure in which an implant surface was decontaminated using a rotary titanium brush and the site of bone defect was restored using a bone graft, all implants were well maintained with stable clinical and periapical radiographic findings throughout the two-year follow-up period.
Go to :





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download