C. albicans causes oral candidiasis, which is especially common in immune compromised patients or the elderly.
14,15 Also, denture stomatitis occurs in people who wear dentures and is associated with
C. albicans.
16 The elderly population is gradually increasing due to the development of medical technology and equipment. This phenomenon can be expected to increase the use of dentures. Therefore, the importance of denture hygiene is emphasized, and the need for a more effective denture cleanser is emerging. This study was investigated antifungal activity of extract from shiitake mushroom to prevent oral candidiasis.
Shiitake mushroom is used general food and is recognized medical value due to its nutritional components. This mushroom has bio-active polysaccharide (lentinan, heteroglucan, xylomannan, and β-glucan), free sugar (arabinose, arabitol, glycerol, mannitol, mannose, and trehalose), vitamins (B2, B12, C, D and E), organic acid (cinnamic acid, phenolic acid, and benzoic acid), and fiber.
17,18 These components of shiitake mushroom show antitumor, antiinflammation, antioxidant and antimicrobial activity.
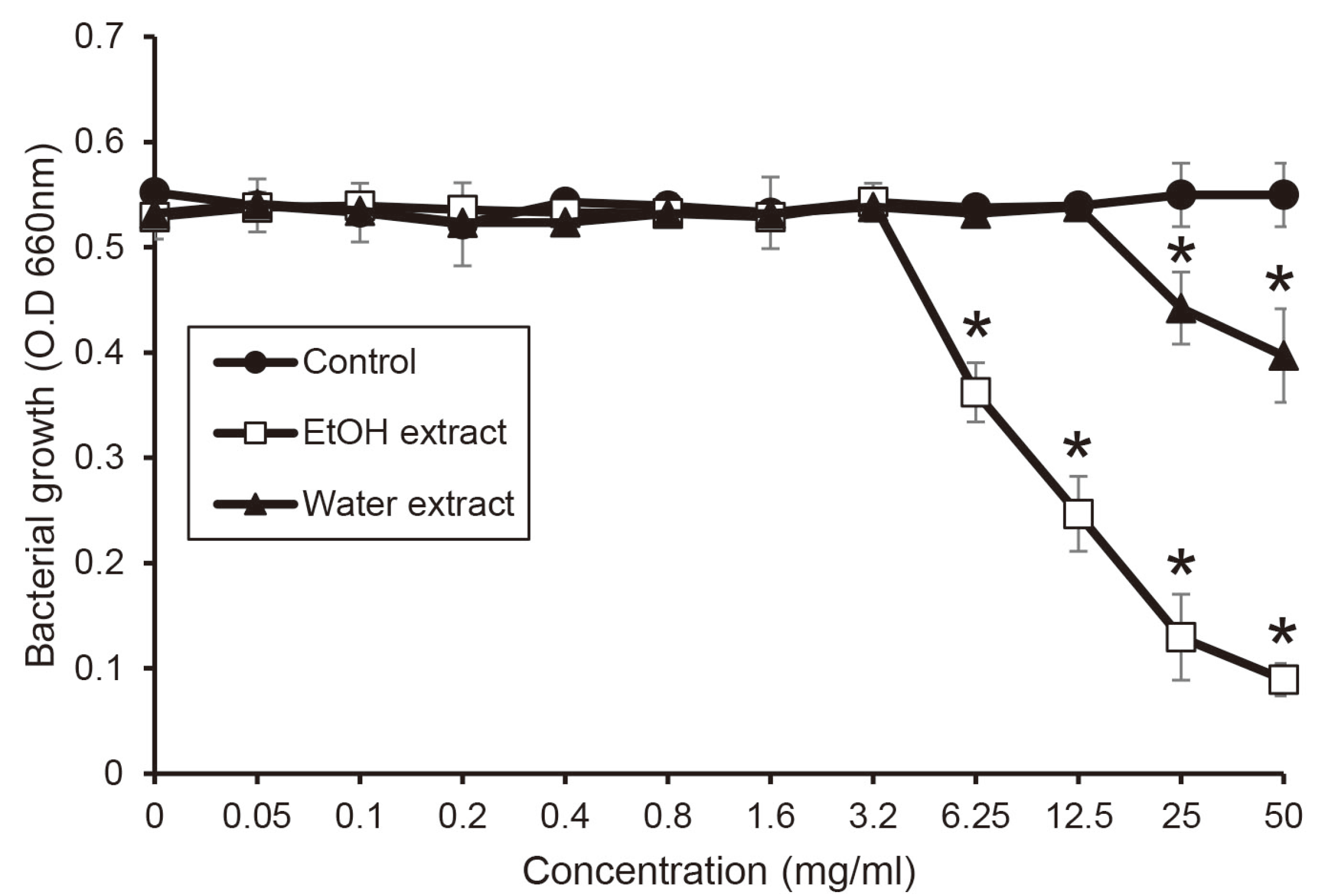
1-3,17 When extract from shiitake mushroom using water and ethanol was investigated antifungal activity against
C. albicans, the ethanol extract showed stronger antifungal activity compared to the water extract. These results indicates that hydrophobic components of shiitake mushroom may be suitable to prevent oral candidiasis. Also, comparing other studies. Mushroom extracts with antifungal activity were reported as organic acid such as benzoic acid, cinnamic acid, and phenolic acid, which is consistent with the results of this study.
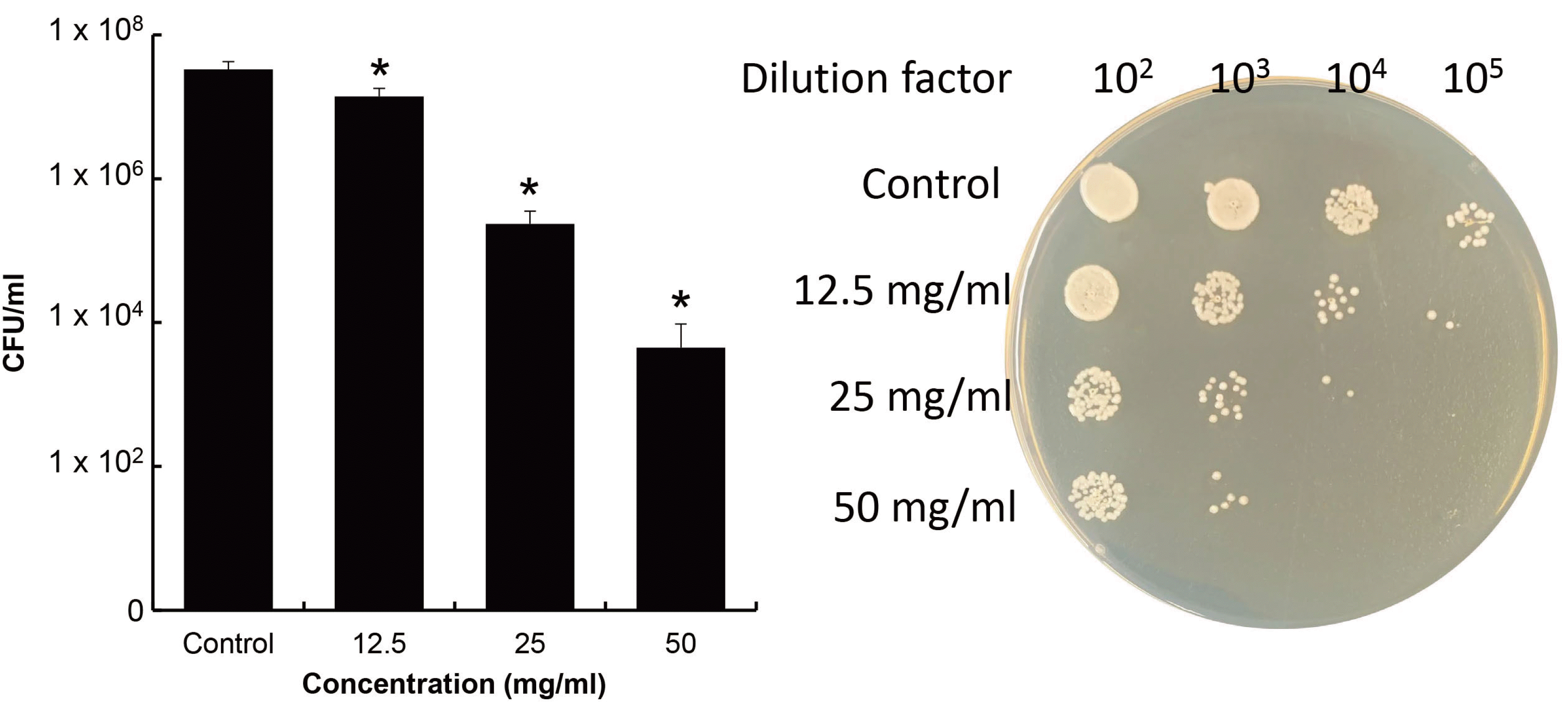
18 Basis of these results, the ethanol extract was investigated antifungal activity against hyphal type of
C. albicans. Since the hyphal type forms biofilm,
C. albicans was formed biofilm on 12-well plate to investigated antifungal activity against hyphal type. The ethanol extract showed antifungal activity against hyphal
C. albicans. In this study, hyphal
C. albicans was evaluated for two reasons. First, the hyphal type forms a biofilm, which forms a protective barrier on the outside of the biofilm with exopolysaccharide.
19 The protective barrier makes it resistant to antifungal agents, and the resistance to antifungal agents is greater than that of the yeast type. Another reason is that
C. albicans mainly exists as a hyphal type in oral cavity. Therefore, in order to investigate the antifungal effects in clinical area, it is necessary to use the hyphal type of
C. albicans. Recently, resistance to antifungal agents of human disease-related fungi is increasing, and thus a treatment is being sought using antifungal agents in crop.
20,21 Shiitake mushroom is also a crop and may be a candidate to be an agricultural fungicide.
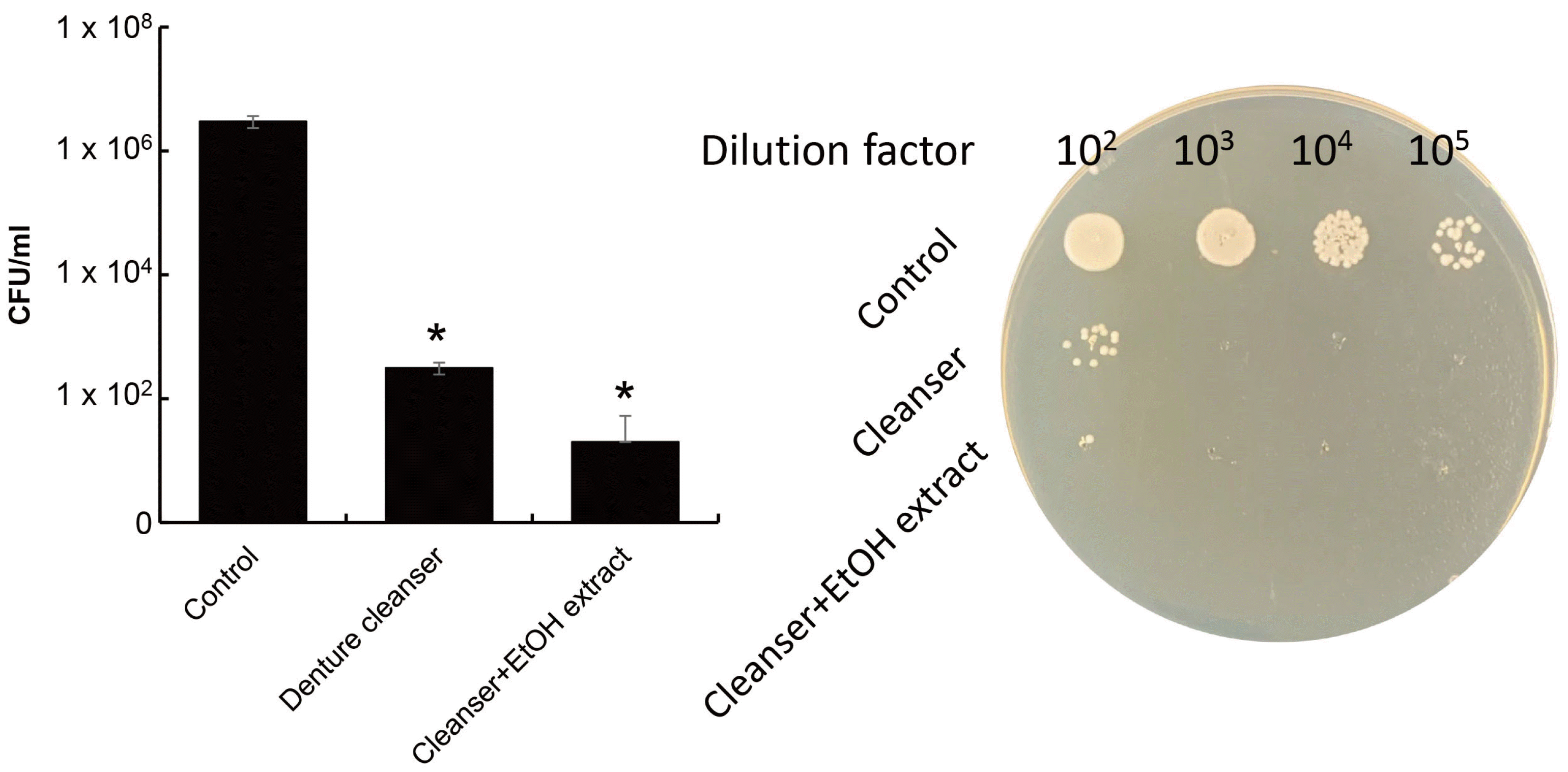
Next, to investigate the effect on denture-related stomatitis, a candidal biofilm was formed on denture resin and its removal ability was tested. Since the antifungal effects of the ethanol extract on candidal biofilm was investigated, the effect when mixed with a denture cleanser was investigated next. The ethanol extract showed a synergistic effect with the denture cleanser on candidal biofilm. This result indicates that the denture cleanser containing enzymes and other constituents had no effect on the ethanol extract. Denture cleanser has been reported to induce oral mucosal injury, chemical burn, and gastric perforation.
22-24 Therefore, it may be safer denture users by reducing or pulling out the strong toxic substances of denture cleanser and using the extract of shiitake mushroom. In addition, a further study is necessary to investigate antifungal activity against candidal biofilm on denture base resin by using the extract of shiitake mushroom and changing the composition of denture cleanser.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download