Abstract
Materials and Methods
The experiment was performed using 7 types of anaerobic bacteria present in the periodontal pocket. The bacteria were cultured using suitable medium in an anaerobic condition with or without hemin, and the growth of the bacteria was measured every 6 hours by a spectrophotometer.
Go to : 
Periodontal disease is an inflammatory disease in oral cavity and related with gram-negative anaerobic bacteria.1 In the 19th century, Socranskii group reported that Porphyromonas gingivalis, Tannerella forsythia (formerly Bacteroides forsythus), and Treponema denticola are closely related with periodontitis by epidemiological study.2 After these results, the researchers studied the virulence factors associated with periodontitis in each bacterium.3 Recently, ecological plaque hypothesis was raised, by which the studies on periodontitis-related bacteria have also changed a lot.4 According to this hypothesis, the induction of periodontitis is related with dysbiosis of periodontal pocket. In healthy people, microbial ecosystem is balanced by Gram-positive facultative anaerobes like normal flora. However, the microbial ecosystem is broken by changes in external or oral environment, and because of this, the number of Gramnegative obligated anaerobes is increased. Eventually, multi-species pathogens may induce periodontitis. Therefore, the studies on effects of complex bacteria rather than single-species bacteria in periodontitis are being investigated.5 Regarding changes in the oral environment, A study was recently reported that gingipain of P. gingivalis induces initial inflammation and increases the influx of gingival crevicular fluid into gingival pocket.6 These symptoms can be expected to increase the proportion of Gram-negative obligated anaerobes.
Heme consists of a tetrapyrrole ring with an iron atom in center. Also, heme is linked specific protein such as hemoglobin, myoglobin, hemopexin, albumin and cytochromes7,8 and reversible binds oxygen and transport electrons in human body. Further more, heme is a damage-associated molecular pattern (DAMP) and triggers inflammatory responses of immune cells.9 Iron is found in the form of heme10 and a source for bacterial growth.11 Periodontitis-related bacteria are also used iron for metabolism, toxicity, and pathogenesis.12-15 In a clinical study, when hemin concentration between healthy and periodontal disease site was compared, the heme in healthy site and the disease site showed range of 0 - 251 nM and 0 - 7723.5 nM, respectively.16 This data makes that it possible to investigate the effects of hemin on relation with periodontitis. However, the effect of hemin on bacterial growth in periodontal pocket has not been reported.
In this study, the bacteria related with periodontitis was investigated growth in various concentration of hemin.
Go to : 
In this study, Filifactor alocis ATCC 35896, Fusobacterium nucleatum ATCC 25586, Porphyromonas gingivalis ATCC 33277, Prevotella intermedia ATCC 25611, Prevotella nigrescens ATCC 33563, Tannerella forsythia ATCC 43037, and Treponema denticola ATCC 35405 were used to investigate effect of hemin. For cultivation of periodontitis-related pathogens, F. alocis was used columbia broth supplemented with yeast extract, L-cysteine (0.2%), L-arginine (0.4%), hemin (5 μg/ml), and vitamin K (0.2 μg/ml). T. forsythia was used modified NOS medium supplemented with N-acetylmuramic acid (0.1 μg/ml), vitamin K (0.2 μg/ml), hemin (5 μg/ml), and fetal bovine serum (4%). T. denticola was used GYGVS medium. F. nucleatum, P. gingivalis, P. intermedia, and P. nigrescens were used brain heart infusion broth supplemented with hemin (5 μg/ml) and vitamin K (0.2 μg/ml). The bacteria were cultured at 37°C in anaerobic condition (H2 5%, CO2 10%, and N2 85%)
Hemin was purchased from sigmaaldrich and extracted from bovine. Hemin stock solutions were prepared in three concentrations as 0.5, 5, and 50 mg/ml. 5, 50 and 500 mg of hemin were completely dissolved with 0.1, 0.5, and 1 ml of 1 N NaOH in conical tube (SPL LifeSciences, Pocheon, Korea), respectively, and distilled water was then filled onto 10 ml. The prepared solution was filtered with polyvinylidene fluoride (PVDF) filter (0.22 μm of pore size). The stock solution was stored in anaerobic chamber before use.
Each cultured bacterium was harvested by centrifugation at 5,000 × g for 10 min, and fresh medium without hemin was added after removing supernatant. The bacteria were counted with bacterial counting chamber (and adjusted with fresh medium without hemin at 1 × 107 cells/ml. Each medium without hemin (9 ml) was added hemin at final concentration of 0.5, 5, 50 and 500 μg/ml using the stock solution, and then the prepared bacteria were inoculated in the medium. The bacterial growth was measured every 6 hours by optical density using a spectrophotometer at 660 nm of wavelength.
Statistical analysis was performed by IBM SPSS statistics ver. 23 software (IBM, Armonk, USA). The data from the different groups was analyzed by the non-parametric Kruskal-Wallis test. Statistical significance was defined by P value of less than 0.05. All data are expressed as the median and interquartile range.
Go to : 
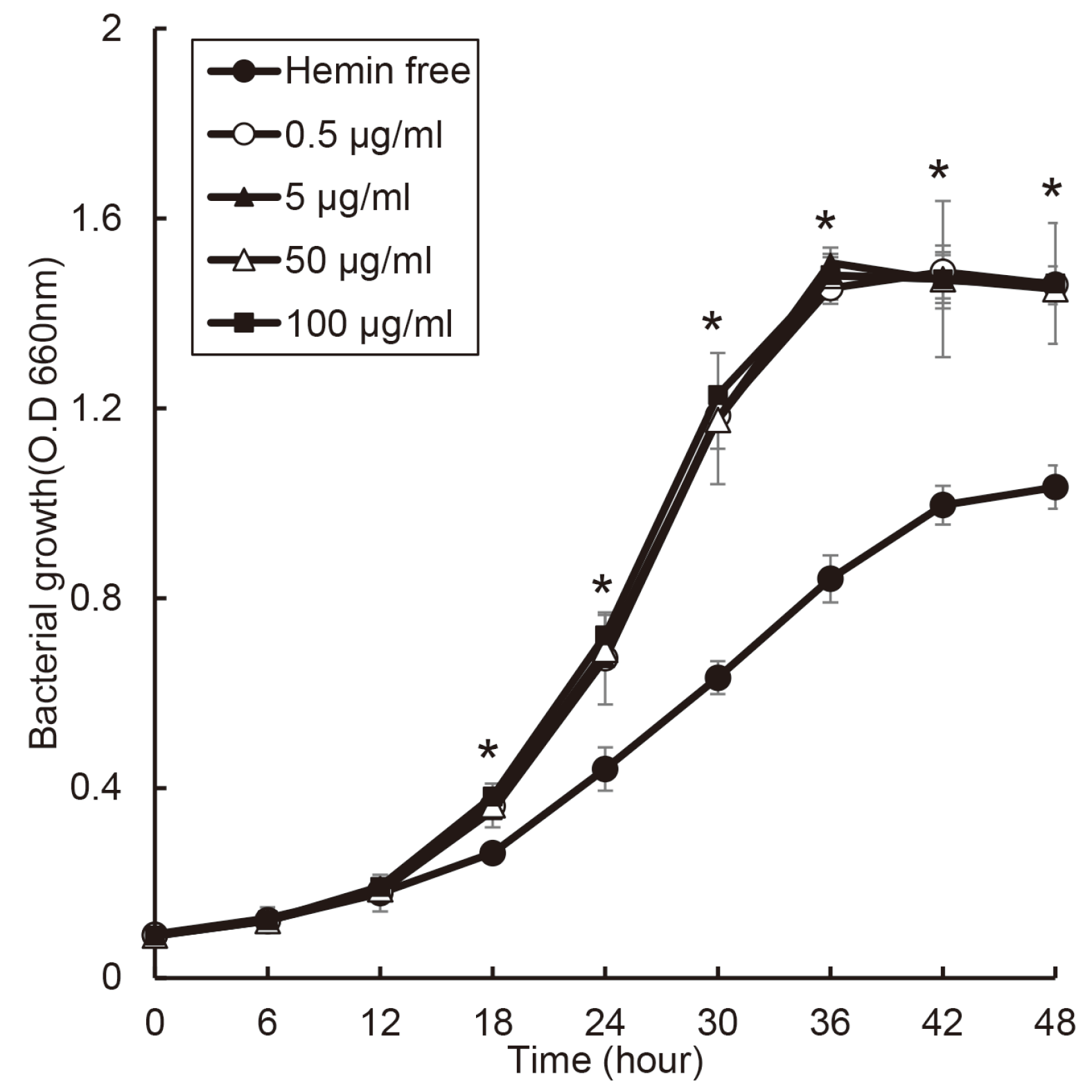
When P. gingivalis was cultured BHI broth including vitamin K with various concentration of hemin, the growth of P. gingivalis did not showed significant difference in the condition with hemin (Fig. 1), and in hemin free condition, P. gingivalis growth was showed slower. The growth of P. gingivalis entered the exponential phase after 12 hours, and reached the stationary phase after 36 hours in all concentration of hemin.
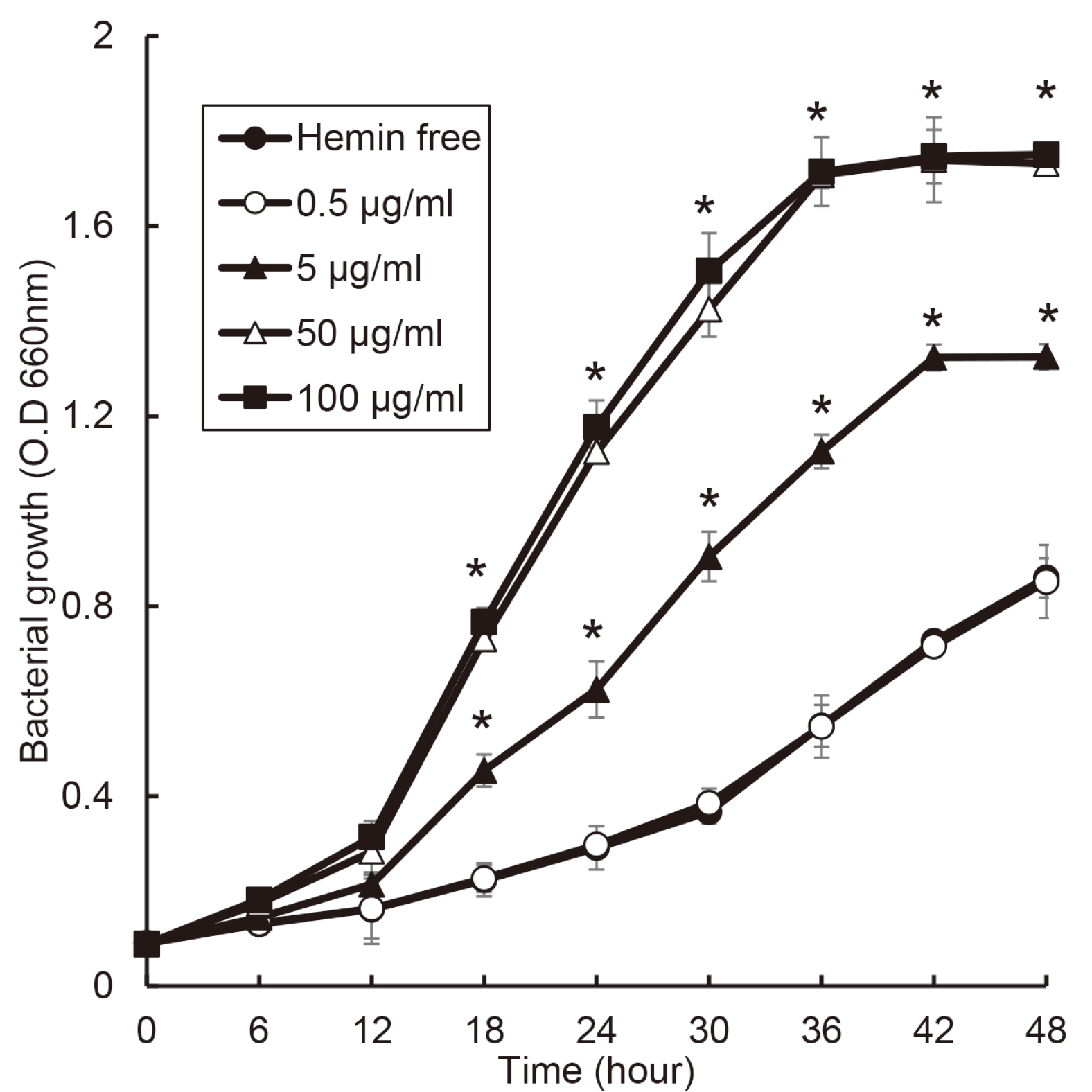
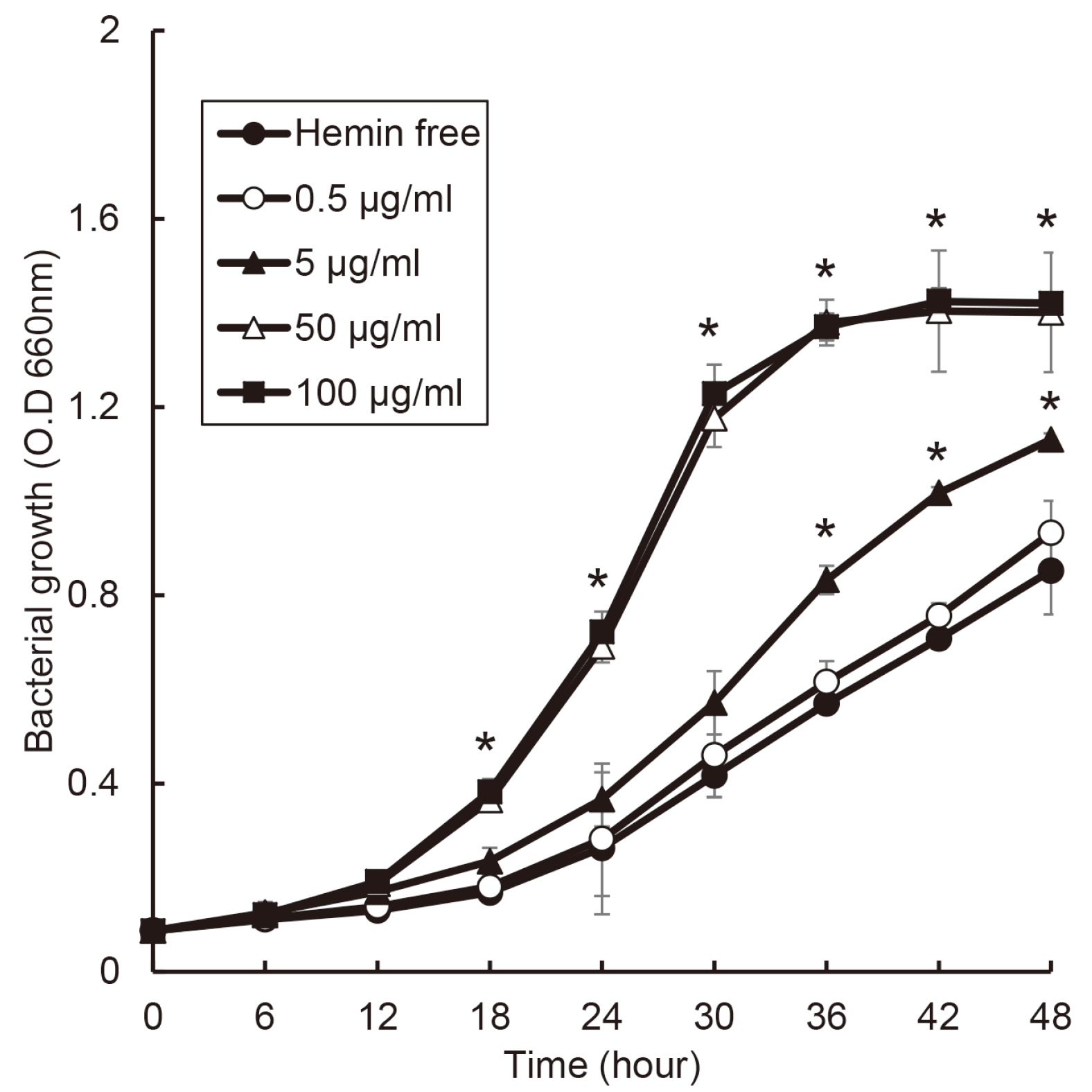
P. nigrescens grew the fastest at 50 and 100 μg/ml of hemin condition, and the growth ratio of P. nigrescens was changed in a dose-dependent manner of hemin (Fig. 2). Furthermore, the stationary phase of P. nigrescens growth showed different concentration by hemin concentration. In case of P. nigrescens, the its growth reached the stationary phase after 42 hours, and P. nigrescens cultured at 0 and 0.5 μg/ml of hemin did not reach stationary phase.
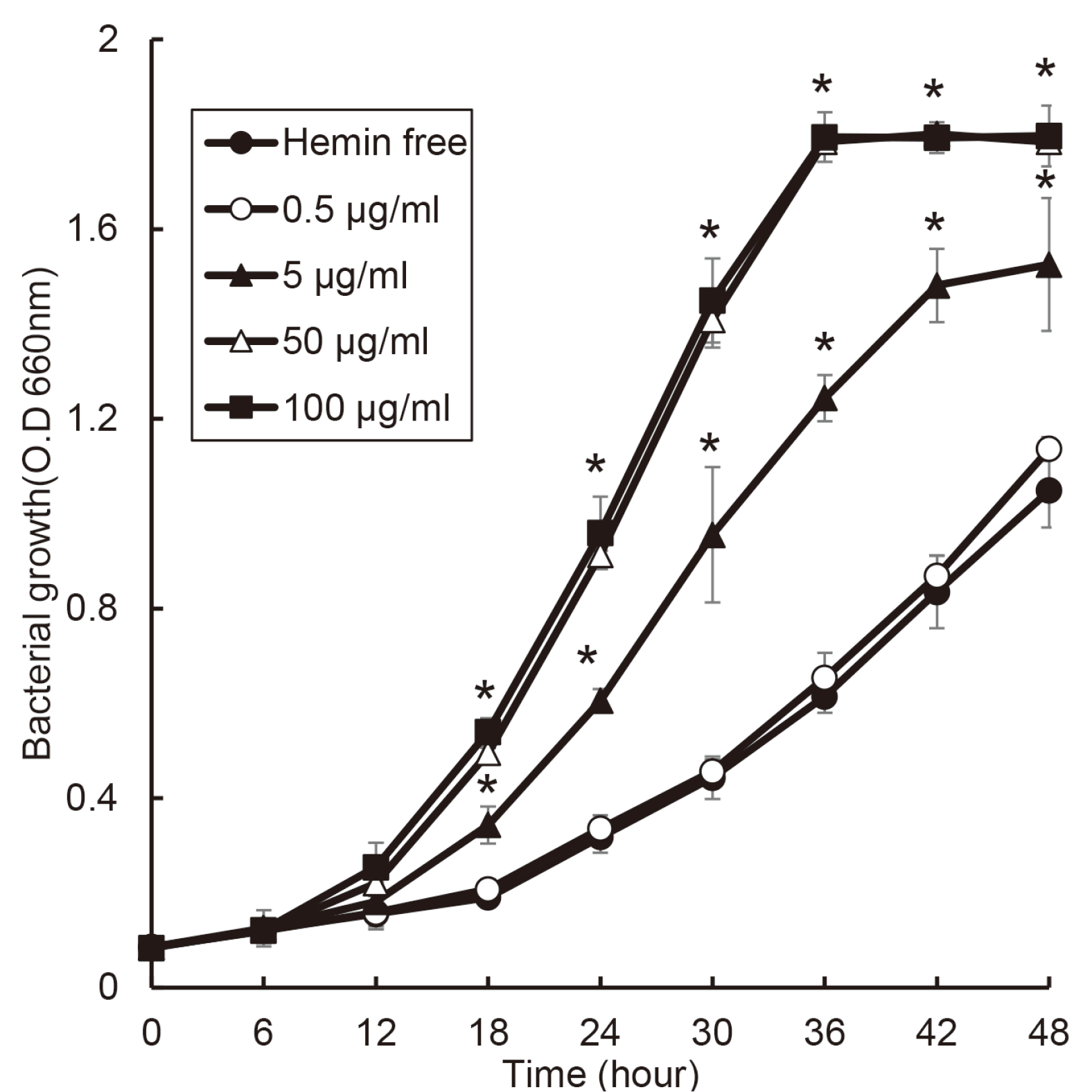
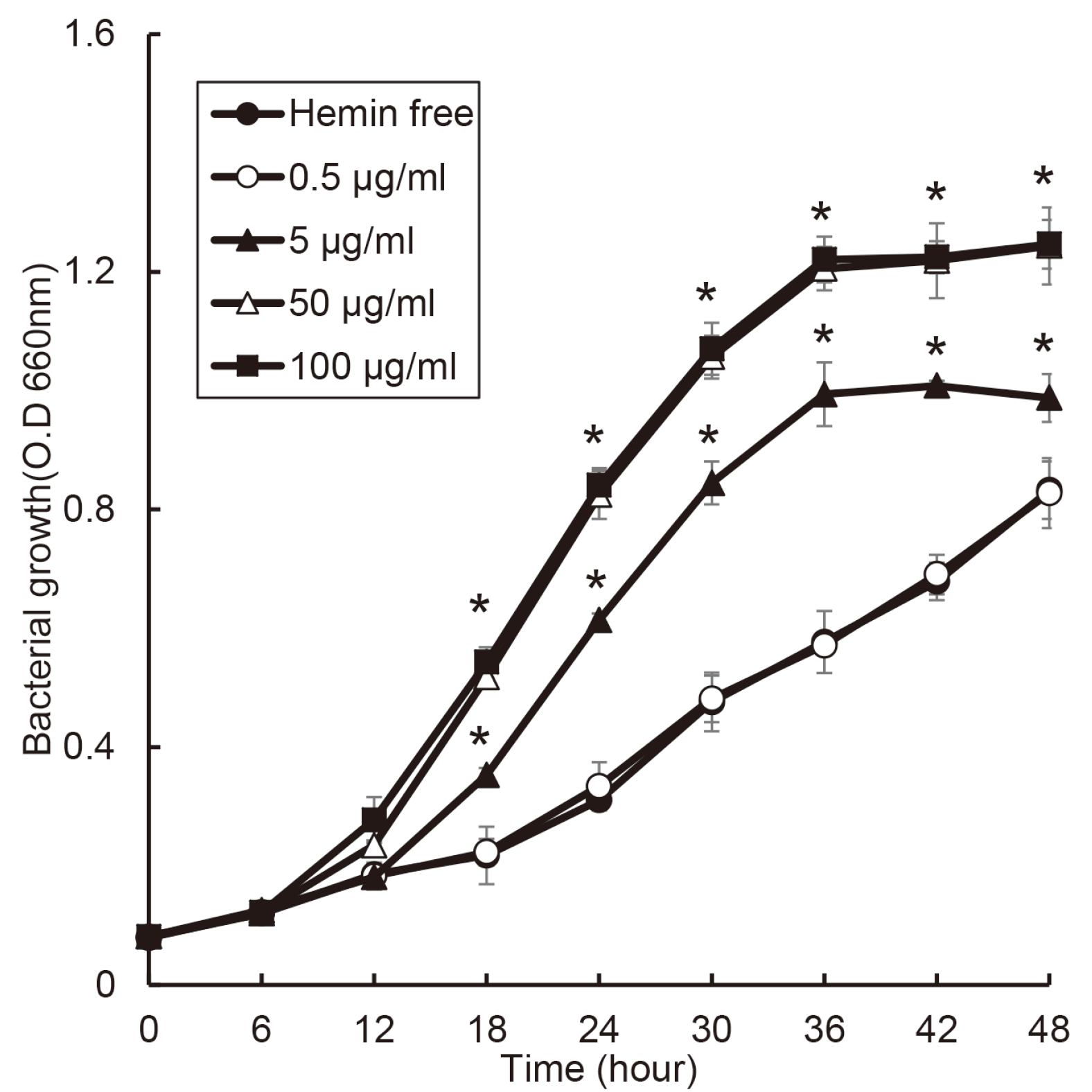
The growth of P. intermedia was changed by hemin concentration (Fig. 3). In the condition of 50 and 100 μg/ml of hemin, the growth curve showed typical S shape such as log, exponential, and stationary phase. P. intermedia entered stationary phase after 36 hours in 50 and 100 μg/ml of hemin and after 42 hours in 5 μg/ml of hemin.
F. nucleatum slowly grew at 0.5 μg/ml of hemin and hemin free condition. and the growth ratio of F. nucleatum was changed in a dose-dependent manner of hemin (Fig. 4). F. nucleatum entered stationary phase after 36 hours in 50 and 100 μg/ml of hemin and after 48 hours in 5 μg/ml of hemin.
F. alocis entered stationary phase between 30 and 36 h after inoculation into fresh medium at 50 and 100 μg/ml of hemin (Fig. 5). F. alocis slowly grew under 5 μg/ml of hemin and did not showed stationary phase to 48 hours. Up concentration of 50 μg/ml of hemin, the growth of F. alocis entered exponential phase after 12 hours and reached stationary phase after 36 hours.
T. forsythia slowly grew under concentration of 5 μg/ml of hemin as shown Fig. 6. The growth of T. forsythia entered exponential phase between 6 and 12 hours and reached stationary phase after 36 hours in 50 and 100 μg/ml of hemin condition. Especially, comparing 50 and 5 μg/ml of hemin condition, the growth of T. forsythia was the same time to reach stationary phase. However, the bacterial concentration was different.
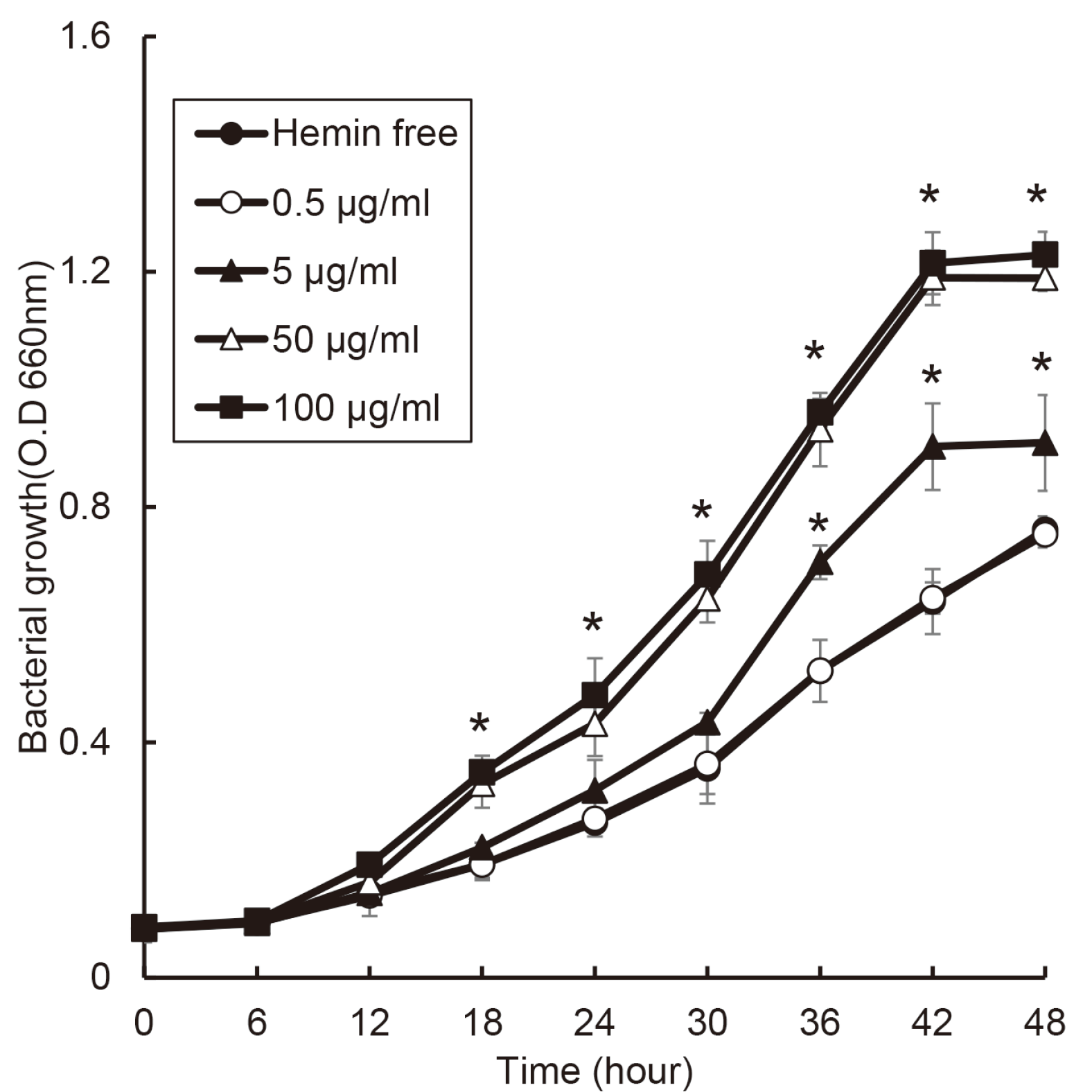
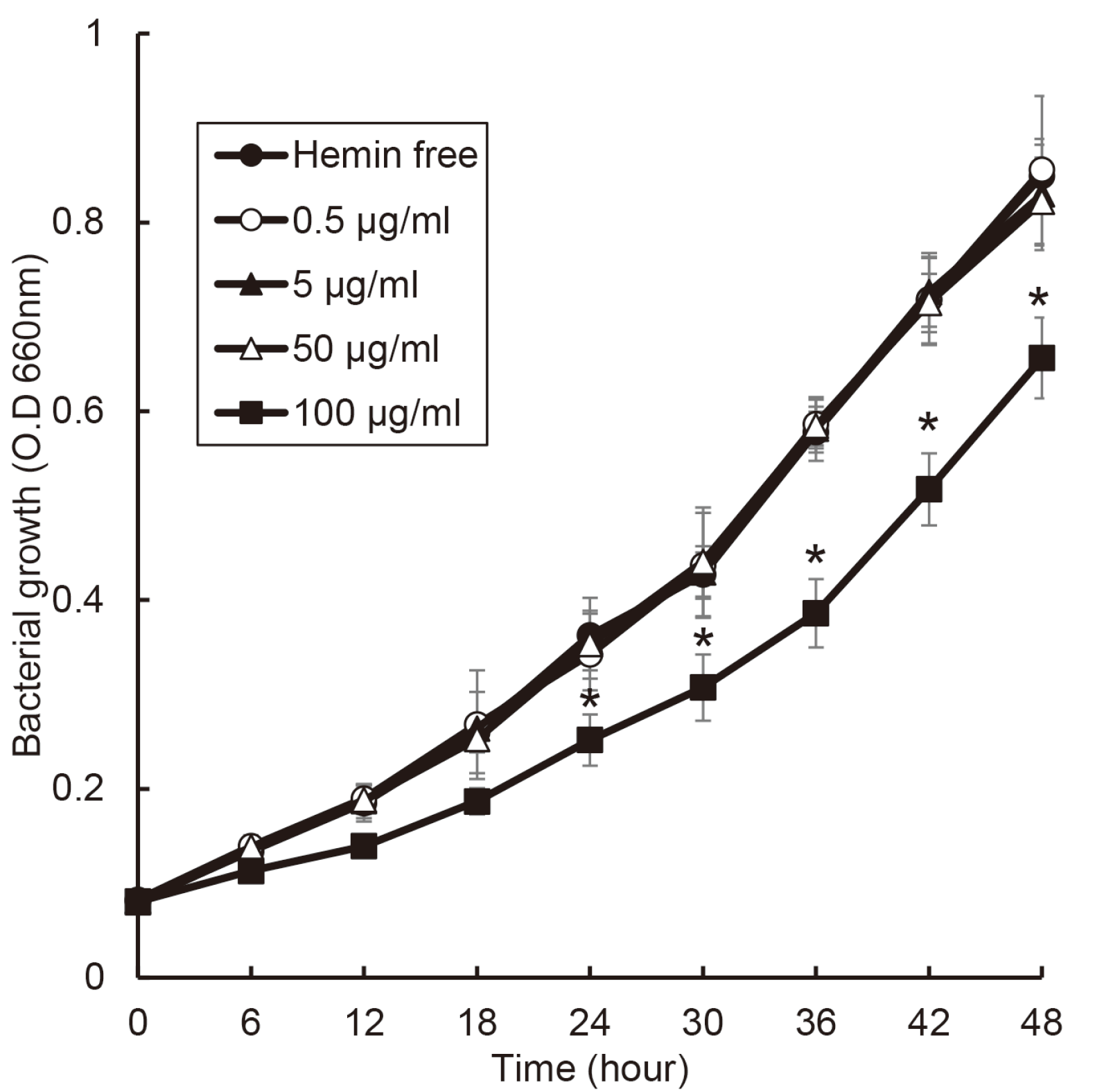
The growth of T. denticola was not affect to 50 μg/ml of hemin concentration. Unlike other bacteria, the growth rate of T. denticola decreased at a concentration of 100 μg/ml of hemin (Fig. 7). At all concentration of hemin, the growth of T. denticola did not reach stationary phase util 48 hours.
Go to : 
In relation of the outbreak of periodontitis, the type of bacteria is previously considered more important, but in recent years, not only the type of bacteria but also the number of total bacteria is considered important. In the 1970s, a specific plaque hypothesis was suggested that specific bacteria were associated with periodontitis,17 and later, Socranskii group suggested P. gingivalis, T. forsythia, and T. denticola as periodontitis-causing bacteria.18 Philip D. March proposed ecological plaque hypothesis that combined specific pathogens and bacterial composition.4 This hypothesis is that oral diseases are the result of an imbalance in the total microflora due to ecological stress, resulting in an enrichment of oral pathogens as periodontitis related bacteria. Also, according to the recent “The Keystone-Pathogen Hypothesis”, P. gingivalis induces initial inflammation of periodontal Fig. 7. Effect of hemin concentration on the growth of T. denticola. T. denticola was cultured with various concentration of hemin or without hemin. Asterisk (*) indicates statistically significant differences compared with hemin free (P < 0.05). tissue using gingipain.6 The initial inflammation increases the influx of gingival crevicular fluid (GCF) into the periodontal pocket and causes dysbiosis in the microbial ecosystem. Therefore, this study was first examined the effect of hemin as a component of GCF on the growth of periodontitis-related bacteria to investigate the factors related to the dysbiosis.
The growth of P. gingivalis was not significantly changed by hemin concentration although the growth was slower in hemin free condition. Except for T. denticola, the growth of anaerobic bacteria present in other periodontal pocket was found to be rapid at certain hemin concentration. In most studies, when cultivating periodontitis-related bacteria, the concentration of hemin is 5 μg/ml.19-21 This concentration is not the best condition for the growth of periodontopathogens. When predicted based on the experimental results, it was found that periodontopathogens grow best at least 50 μg/ml of hemin. In case of P. gingivalis, the optimal condition was when the hemin concentration was 5 μg/ml or higher. Unlike other bacteria, P. gingivalis grew well in low concentration of hemin. In clinical study about measurement of hemin concentration, the average hemin concentration is 46.6 nM in healthy sites and 1116.6 nM in diseased sites.16 Based on this clinical study and the results, it is possible to explain “The Keystone pathogen hypothesis” and “Ecological plaque hypothesis”. P. gingivalis induced initial inflammation, by which GCF is flowed into periodontal pocket and heme concentration may be increased. This elevated heme condition may promote the growth of other periodontopathogens. The heme is a factor that cause dysbiosis in the microbial ecosystem of the periodontal pocket. Eventually, the rapid growth of the bacteria related to periodontitis occupies a large part in the microbial ecosystem and induces periodontitis.
Recently, ecological plaque hypothesis has been recognized as the theory regarding the occurrence of periodontitis. Although it is known that dysbiosis in periodontal pocket occurs due to the influx of GCF, there is little information about which factors cause the dysbiosis.
Go to : 
The present study showed that periodontopathogens rapidly grow above a specific concentration of hemin. Eventually, heme may be a factor that leads dysbiosis in the microbial ecosystem of the subgingival plaque and thereby promote a periodontitis-causing environment. The further study will be necessary to investigate the change in virulence of periodontopathogens caused by changes in hemin concentration.
Go to : 
References
1. Dahlén GG. 1993; Black-pigmented gram-negative anaerobes in periodontitis. FEMS Immunol Med Microbiol. 6:181–92. DOI: 10.1016/0928-8244(93)90089-M. PMID: 8518755.
2. Socransky SS. 1977; Microbiology of periodontal disease - present status and future considerations. J Periodontol. 48:497–504. DOI: 10.1902/jop.1977.48.9.497. PMID: 333085.
3. O'Brien-Simpson NM, Veith PD, Dashper SG, Reynolds EC. 2004; Antigens of bacteria associated with periodontitis. Periodontol 2000. 35:101–34. DOI: 10.1111/j.0906-6713.2004.003559.x. PMID: 15107060.
4. Marsh PD. 1994; Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res. 8:263–71. DOI: 10.1177/08959374940080022001. PMID: 7865085.
5. Listgarten MA. 1988; The role of dental plaque in gingivitis and periodontitis. J Clin Periodontol. 15:485–7. DOI: 10.1111/j.1600-051X.1988.tb01019.x. PMID: 3053789.
6. Hajishengallis G, Darveau RP, Curtis MA. 2012; The keystone-pathogen hypothesis. Nat Rev Microbiol. 10:717–25. DOI: 10.1038/nrmicro2873. PMID: 22941505. PMCID: PMC3498498.
7. Wijnsma KL, Veissi ST, de Wijs S, van der Velden T, Volokhina EB, Wagener FADTG, van de Kar NCAJ, van den Heuvel LP. 2020; Heme as Possible Contributing Factor in the Evolvement of Shiga-Toxin Escherichia coli Induced Hemolytic-Uremic Syndrome. Front Immunol. 11:547406. DOI: 10.3389/fimmu.2020.547406. PMID: 33414780. PMCID: PMC7783363.
8. Muller-Eberhard U, Fraig M. 1993; Bioactivity of heme and its containment. Am J Hematol. 42:59–62. DOI: 10.1002/ajh.2830420112. PMID: 8416298.
9. Chaudhry SR, Hafez A, Rezai Jahromi B, Kinfe TM, Lamprecht A, Niemela M, Muhammad S. 2018; Role of Damage Associated Molecular Pattern Molecules (DAMPs) in Aneurysmal Subarachnoid Hemorrhage (aSAH). Int J Mol Sci. 19:2035. DOI: 10.3390/ijms19072035. PMID: 30011792. PMCID: PMC6073937.
10. Liu X, Olczak T, Guo HC, Dixon DW, Genco CA. 2006; Identification of amino acid residues involved in heme binding and hemoprotein utilization in the Porphyromonas gingivalis heme receptor HmuR. Infect Immun. 74:1222–32. DOI: 10.1128/IAI.74.2.1222-1232.2006. PMID: 16428772. PMCID: PMC1360300.
11. Rouault TA. 2004; Microbiology. Pathogenic bacteria prefer heme. Science. 305:1577–8. DOI: 10.1126/science.1102975. PMID: 15361615.
12. Al-Qutub MN, Braham PH, Karimi-Naser LM, Liu X, Genco CA, Darveau RP. 2006; Hemin-dependent modulation of the lipid A structure of Porphyromonas gingivalis lipopolysaccharide. Infect Immun. 74:4474–85. DOI: 10.1128/IAI.01924-05. PMID: 16861633. PMCID: PMC1539574.
13. Lee HR, Rhyu IC, Kim HD, Jun HK, Min BM, Lee SH, Choi BK. 2011; In-vivo-induced antigenic determinants of Fusobacterium nucleatum subsp. nucleatum. Mol Oral Microbiol. 26:164–72. DOI: 10.1111/j.2041-1014.2010.00594.x. PMID: 21375706.
14. Yu F, Anaya C, Lewis JP. 2007; Outer membrane proteome of Prevotella intermedia 17: identification of thioredoxin and iron-repressible hemin uptake loci. Proteomics. 7:403–12. DOI: 10.1002/pmic.200600441. PMID: 17177252.
15. Aguilera O, Andrés MT, Heath J, Fierro JF, Douglas CW. 1998; Evaluation of the antimicrobial effect of lactoferrin on Porphyromonas gingivalis, Prevotella intermedia and Prevotella nigrescens. FEMS Immunol Med Microbiol. 21:29–36. DOI: 10.1111/j.1574-695X.1998.tb01146.x. PMID: 9657318.
16. Liu LY, McGreor N, Wong BK, Butt H, Darby IB. 2016; The association between clinical periodontal parameters and free haem concentration within the gingival crevicular fluid: a pilot study. J Periodontal Res. 51:86–94. DOI: 10.1111/jre.12286. PMID: 26094689.
17. Loesche WJ. 1976; Chemotherapy of dental plaque infections. Oral Sci Rev. 9:65–107. PMID: 1067529.
18. Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr. 1998; Microbial complexes in subgingival plaque. J Clin Periodontol. 25:134–44. DOI: 10.1111/j.1600-051X.1998.tb02419.x. PMID: 9495612.
19. Olczak T, Simpson W, Liu X, Genco CA. 2005; Iron and heme utilization in Porphyromonas gingivalis. FEMS Microbiol Rev. 29:119–44. DOI: 10.1016/j.femsre.2004.09.001. PMID: 15652979.
20. Lee SH, Baek DH. 2014; Effects of Streptococcus thermophilus on volatile sulfur compounds produced by Porphyromonas gingivalis. Arch Oral Biol. 59:1205–10. DOI: 10.1016/j.archoralbio.2014.07.006. PMID: 25105253.
21. Lee SH, Baek DH. 2013; Characteristics of Porphyromonas gingivalis lipopolysaccharide in co-culture with Fusobacterium nucleatum. Mol Oral Microbiol. 28:230–8. DOI: 10.1111/omi.12020. PMID: 23347381.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download