1. Lang NP, Berglundh T. Periimplant diseases:where are we now?-Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol. 2011; 38(Suppl 11):178–81. DOI:
10.1111/j.1600-051X.2010.01674.x. PMID:
21323713.
2. Sgolastra F, Petrucci A, Severino M, Gatto R, Monaco A. Periodontitis, implant loss and peri-implantitis. A meta-analysis. Clin Oral Implants Res. 2015; 26:e8–e16. DOI:
10.1111/clr.12319. PMID:
24382358.
3. Dalago HR, Schuldt Filho G, Rodrigues MA, Renvert S, Bianchini MA. Risk indicators for Peri-implantitis. A cross-sectional study with 916 implants. Clin Oral Implants Res. 2017; 28:144–50. DOI:
10.1111/clr.12772. PMID:
26754342.
4. Serino G, Strom C. Peri-implantitis in partially edentulous patients:association with inadequate plaque control. Clin Oral Implants Res. 2009; 20:169–74. DOI:
10.1111/j.1600-0501.2008.01627.x. PMID:
19077152.
5. Zhuang LF, Watt RM, Mattheos N, Si MS, Lai HC, Lang NP. Periodontal and peri-implant microbiota in patients with healthy and inflamed periodontal and peri-implant tissues. Clin Oral Implants Res. 2016; 27:13–21. DOI:
10.1111/clr.12508. PMID:
25399962.
6. Ximenez-Fyvie LA, Haffajee AD, Socransky SS. Comparison of the microbiota of supra- and subgingival plaque in health and periodontitis. J Clin Periodontol. 2000; 27:648–57. DOI:
10.1034/j.1600-051x.2000.027009648.x. PMID:
10983598.
8. Kolenbrander PE, Palmer RJ Jr, Periasamy S, Jakubovics NS. Oral multispecies biofilm development and the key role of cell-cell distance. Nat Rev Microbiol. 2010; 8:471–80. DOI:
10.1038/nrmicro2381. PMID:
20514044.
10. Perez-Chaparro PJ, Duarte PM, Shibli JA, Montenegro S, Lacerda Heluy S, Figueiredo LC, Faveri M, Feres M. The current weight of evidence of the microbiologic profile associated with peri-implantitis:A systematic review. J Periodontol. 2016; 87:1295–304. DOI:
10.1902/jop.2016.160184. PMID:
27420109.
11. Scarano A, Piattelli M, Caputi S, Favero GA, Piattelli A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks:an in vivo human study. J Periodontol. 2004; 75:292–6. DOI:
10.1902/jop.2004.75.2.292. PMID:
15068118.
12. Holst S, Blatz MB, Hegenbarth E, Wichmann M, Eitner S. Prosthodontic considerations for predictable single-implant esthetics in the anterior maxilla. J Oral Maxillofac Surg. 2005; 63:89–96. DOI:
10.1016/j.joms.2005.05.161. PMID:
16125019.
13. Chen D, Wang N, Gao Y, Shao L, Deng B. A 3-dimensional finite element analysis of the restoration of the maxillary canine with a complex zirconia post system. J Prosthet Dent. 2014; 112:1406–15. DOI:
10.1016/j.prosdent.2014.05.017. PMID:
24993379.
14. Heuer W, Elter C, Demling A, Neumann A, Suerbaum S, Hannig M, Heidenblut T, Bach FW, Stiesch-Scholz M. Analysis of early biofilm formation on oral implants in man. J Oral Rehabil. 2007; 34:377–82. DOI:
10.1111/j.1365-2842.2007.01725.x. PMID:
17441878.
15. Nascimento CD, Pita MS, Fernandes FHNC, Pedrazzi V, de Albuquerque Junior RF, Ribeiro RF. Bacterial adhesion on the titanium and zirconia abutment surfaces. Clin Oral Implants Res. 2014; 25:337–43. DOI:
10.1111/clr.12093. PMID:
23316996.
16. Rimondini L, Fare S, Chiesa R, Pedeferri MP, Carrassi A. The effect of composition, wettability and roughness of the substrate on in vivo early bacterial colonization of titanium. J Appl Biomater Biomech. 2003; 1:131–8. PMID:
20803464.
17. Subramani K, Jung RE, Molenberg A, Hammerle CH. Biofilm on dental implants:a review of the literature. Int J Oral Maxillofac Implants. 2009; 24:616–26. PMID:
19885401.
18. Renvert S, Roos-Jansaker AM, Claffey N. Non-surgical treatment of peri-implant mucositis and peri-implantitis:a literature review. J Clin Periodontol. 2008; 35:305–15. DOI:
10.1111/j.1600-051X.2008.01276.x. PMID:
18724858.
19. Wainwright M. Photodynamic antimicrobial chemotherapy (PACT). J Antimicrob Chemother. 1998; 42:13–28. DOI:
10.1093/jac/42.1.13. PMID:
9700525.
20. Wood S, Metcalf D, Devine D, Robinson C. Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms. J Antimicrob Chemother. 2006; 57:680–4. DOI:
10.1093/jac/dkl021. PMID:
16464894.
21. Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998; 90:889–905. DOI:
10.1093/jnci/90.12.889. PMID:
9637138. PMCID:
PMC4592754.
22. Bassetti M, Schar D, Wicki B, Eick S, Ramseier CA, Arweiler NB, Sculean A, Salvi GE. Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy:12-month outcomes of a randomized controlled clinical trial. Clin Oral Implants Res. 2014; 25:279–87. DOI:
10.1111/clr.12155. PMID:
23560645.
23. Vohra F, Al-Rifaiy MQ, Lillywhite G, Abu Hassan MI, Javed F. Efficacy of mechanical debridement with adjunct antimicrobial photodynamic therapy for the management of peri-implant diseases:a systematic review. Photochem Photobiol Sci. 2014; 13:1160–8. DOI:
10.1039/C4PP00083H. PMID:
24924586.
24. Wang H, Li W, Zhang D, Li W, Wang Z. Adjunctive photodynamic therapy improves the outcomes of peri-implantitis:a randomized controlled trial. Aust Dent J. 2019; 64:256–62. DOI:
10.1111/adj.12705. PMID:
31152567.
25. Lee H, Kim YG, Um HS, Chang BS, Lee SY, Lee JK. Efficacy of an LED toothbrush on a Porphyromonas gingivalis biofilm on a sandblasted and acid-etched titanium surface:an in vitro study. J Periodontal Implant Sci. 2018; 48:164–73. DOI:
10.5051/jpis.2018.48.3.164. PMID:
29984046. PMCID:
PMC6031762.
26. Blanc V, Isabal S, Sanchez MC, Llama-Palacios A, Herrera D, Sanz M, Leon R. Characterization and application of a flow system for in vitro multispecies oral biofilm formation. J Periodontal Res. 2014; 49:323–32. DOI:
10.1111/jre.12110. PMID:
23815431.
28. Song WS, Lee JK, Park SH, Um HS, Lee SY, Chang BS. Comparison of periodontitis-associated oral biofilm formation under dynamic and static conditions. J Periodontal Implant Sci. 2017; 47:219–30. DOI:
10.5051/jpis.2017.47.4.219. PMID:
28861286. PMCID:
PMC5577440.
29. Louropoulou A, Slot DE, Van der Weijden F. The effects of mechanical instruments on contaminated titanium dental implant surfaces:a systematic review. Clin Oral Implants Res. 2014; 25:1149–60. DOI:
10.1111/clr.12224. PMID:
23834327.
30. Salvi GE, Ramseier CA. Efficacy of patient-administered mechanical and/or chemical plaque control protocols in the management of peri-implant mucositis. A systematic review. J Clin Periodontol. 2015; 42(Suppl 16):S187–201. DOI:
10.1111/jcpe.12321. PMID:
25495416.
31. Park JB, Koh M, Jang YJ, Choi BK, Kim KK, Ko Y. Removing bacteria from rough surface titanium discs with chlorhexidine and additional brushing with dentifrice. Gerodontology. 2016; 33:28–35. DOI:
10.1111/ger.12106. PMID:
24417576.
32. Schwarz F, John G, Hegewald A, Becker J. Non-surgical treatment of peri-implant mucositis and peri-implantitis at zirconia implants:a prospective case series. J Clin Periodontol. 2015; 42:783–8. DOI:
10.1111/jcpe.12439. PMID:
26249545.
33. Song HH, Lee JK, Um HS, Chang BS, Lee SY, Lee MK. Phototoxic effect of blue light on the planktonic and biofilm state of anaerobic periodontal pathogens. J Periodontal Implant Sci. 2013; 43:72–8. DOI:
10.5051/jpis.2013.43.2.72. PMID:
23678390. PMCID:
PMC3651940.
34. Habiboallah G, Mahdi Z, Mahbobeh NN, Mina ZJ, Sina F, Majid Z. Bactericidal effect of visible light in the presence of erythrosine on Porphyromonas gingivalis and Fusobacterium nucleatum compared with diode laser, an in vitro study. Laser Ther. 2014; 23:263–71. DOI:
10.5978/islsm.14-ER-01. DOI:
10.5978/islsm.14-OR-20. PMID:
25705082. PMCID:
PMC4331568.
35. Metcalf D, Robinson C, Devine D, Wood S. Enhancement of erythrosine-mediated photodynamic therapy of Streptococcus mutans biofilms by light fractionation. J Antimicrob Chemother. 2006; 58:190–2. DOI:
10.1093/jac/dkl205. PMID:
16735434.
36. Røder HL, Raghupathi PK, Herschend J, Brejnrod A, Knøchel S, Sørensen SJ, Burmølle M. Interspecies interactions result in enhanced biofilm formation by co-cultures of bacteria isolated from a food processing environment. Food Microbiol. 2015; 51:18–24. DOI:
10.1016/j.fm.2015.04.008. PMID:
26187823.
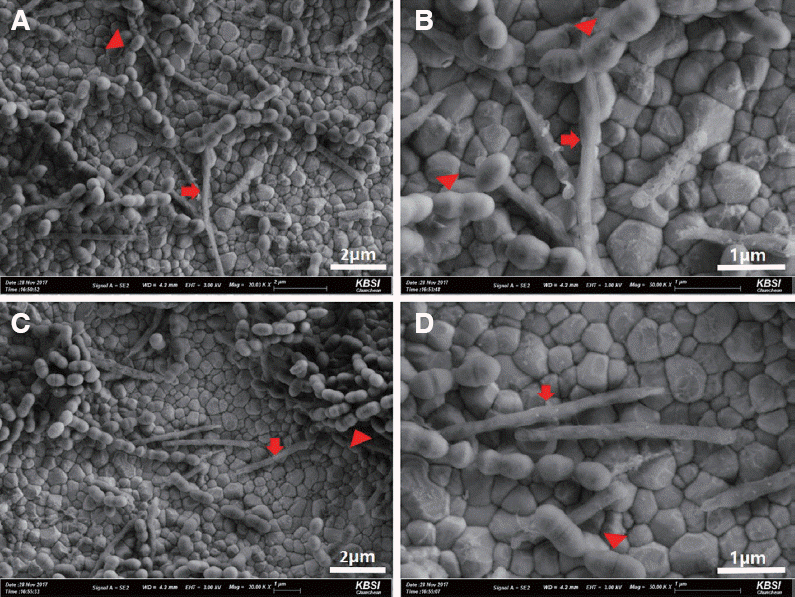
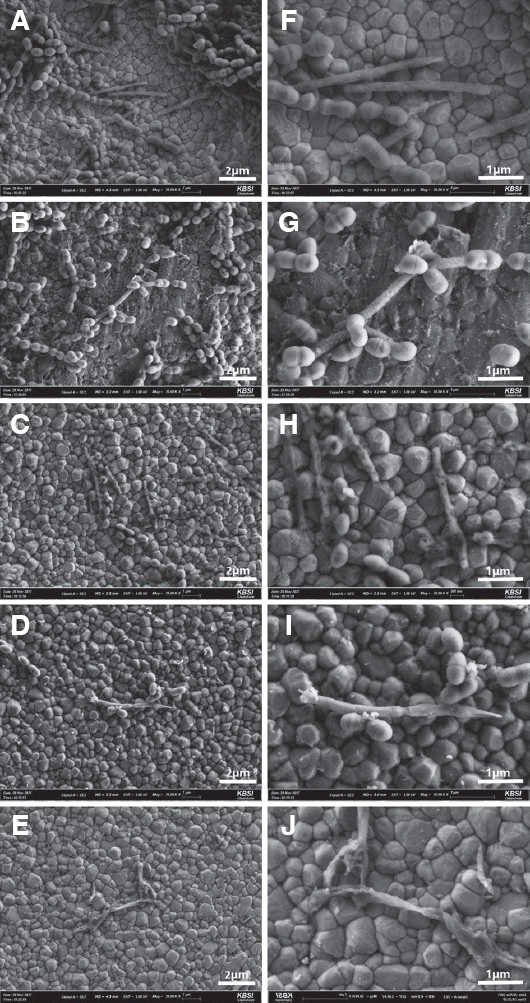




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download