This article has been
cited by other articles in ScienceCentral.
초록
법랑아 세포종은 치성 상피에서 기인한 양성 종양의 일종이다. 하악골에 가장 흔하게 발생하는 양성종양이며 공격적인 성장과 국소적 침범의 특징을 가진다. 그 중 단방성 법랑아 세포종은 방사선학적으로는 단방성의 특징을 가지며 병리학적으로는 낭종의 특징을 가진다. 낭종성 법랑아 세포종의 병소의 크기가 큰 경우 감압술 및 조대술이 보존적인 치료 방법으로 사용된다. 이 치료 방법의 목적은 병소의 크기를 줄여 완전 적출이 손쉽게 하며 악안면 부위 변형이나 신경 손상을 방지하는데 있다. 본 증례에서는 병소의 크기가 큰 낭종성 법랑아 세포종을 감압술 및 조대술로 성공적으로 치료한 치험례를 논문 고찰과 함께 보고하고자 한다.
Go to :

Abstract
Ameloblastoma is a benign neoplasm originating from odontogenic epithelium. It is the most common neoplasm in the jaws and is characterized by aggressive behavior and local invasion. Unicystic ameloblastoma (UA) has a unilocular feature in radiologic examination and a cystic feature histologically. Decompression and marsupialization are conservative method of treatment of large UA. The purpose of decompression and marsupialization are size reduction of the mass, which makes it easy to handle at total enucleation with protection of nerve damage and facial deformity. Here we report successful conservative treatment of extensive UA using decompression and marsupialization with a review of literatures.
Go to :

Keywords: 낭종성 법랑아 세포종, 감압술, 조대술
Keywords: unicystic ameloblastoma, decompression, marsupialization
Introduction
Ameloblastoma is a benign neoplasm originating from odontogenic epithelium.
1 It is the most common neoplasm in the jaws and is characterized by aggressive behavior and local invasion.
2 Intraosseous ameloblastomas are classified into solid, unicystic, and multicystic types.
3 According to the World Health Organization, unicystic ameloblastoma (UA) is similar to a cyst.4 UA has a more favorable prognosis compared to solid and multicystic type and its recurrence rate ranges from 3.6% to 30.5% depending on the treatment method.
2,
5 The highest recurrence rate (30.6%) is observed after simple enucleation, whereas the lowest recurrence rate (3.6%) is observed after resection; a recurrence rate of 18% was reported when marsupialization was used before curettage.
6,
7
Decompression and marsupialization are conservative method of treatment of large cysts and UA.
6-
8 The purpose of decompression and marsupialization are size reduction of the mass, which makes it easy to handle at total enucleation.
6-
8 The success of decompression and marsupialization are influenced by various factors, such as age, surgeon’s technique, amount of incisional biopsy tissue during marsupialization, and close radiological follow-up.
2
Here we report successful conservative treatment of extensive UA using conservative therapy with a review of literature.
Go to :

Case report
A 12-year-old female patient was referred to the Department of Oral and Maxillofacial Surgery office for evaluation of facial swelling and a radiolucentic lesion in the left mandibular area. The swelling started one month ago. She had no medical history. Extraoral examination showed swelling and redness in the left submandibular area. Intraoral examination showed dental caries of #75 and no bleeding or discharging in the oral cavity.
Dental panoramic view showed completely impacted #35 and a unicystic radiolucent lesion in the left mandible (
Fig. 1). Bone expansion and rarefaction was shown in computed tomography (
Fig. 2). Perforation of the buccal and lingual sides of the mandible was also detected (
Fig. 2). The size of the mass was approximately 4.5 cm × 2.6 cm × 2.9 cm (length × width × height). In laboratory analysis, all parameters were within normal ranges.
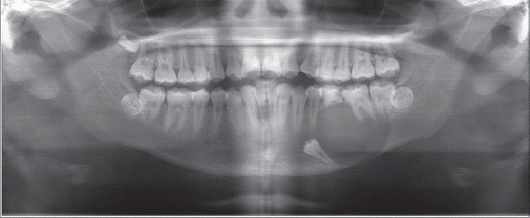 | Fig. 1Dental panoramic view. Large radiolucent lesion including #35 is detected in the left mandible. 
|
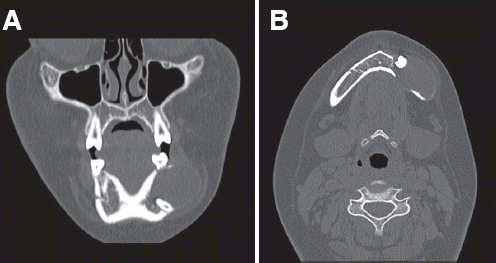 | Fig. 2Computed tomography (CT) view. Perforation of both side of the mandible is clearly detected. (A) Coronal view, (B) Axial view. 
|
Decompression and marsupialization with incisional biopsy were performed under local anesthesia. Resected abnormal tissues and adjacent normal tissues from around the mass were subjected to histological analysis. A penrose drain tube was inserted into the mass to prevent gingival healing (
Fig. 3). Histological diagnosis revealed UA. She had regular intraoral dressing. Six months after decompression and marsupialization, the size of the mass decreased to approximately 2.7 cm × 0.8 cm × 2.0 cm. No perforation of the mandible was detected (
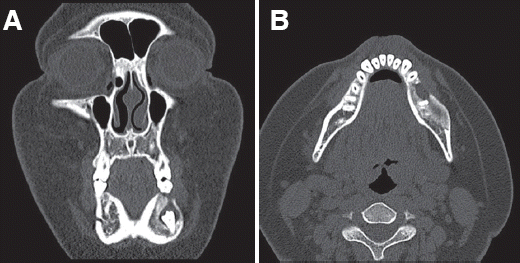
Fig. 4). Eight months after decompression and marsupialization, the mass size further decreased to approximately 2.0 cm × 0.8 cm × 1.5 cm (
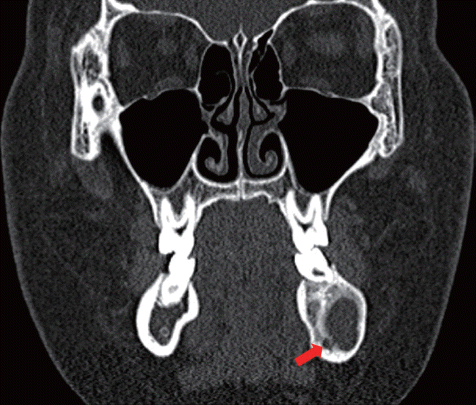
Fig. 5). New bone formation around the inferior alveolar nerve was detected (
Fig. 6). Resection of the benign mass with surgical extraction of #35 and #75 was performed under general anesthesia. The resected mass was subjected to histological analysis and the final diagnosis was confirmed as UA (
Fig. 7). After surgery, she had no hypoesthesia of the left chin and lower lip. After follow-up for 2 years, she had no recurrence of ameloblastoma and complete bone healing (
Fig. 8). She is scheduled for orthodontic treatment.
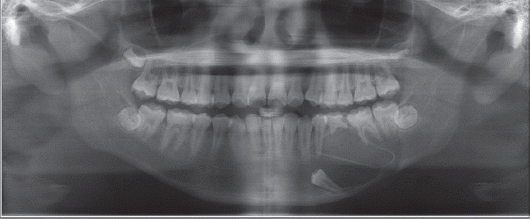 | Fig. 3Post operative dental panoramic view. A penrose drain is inserted into the mass. 
|
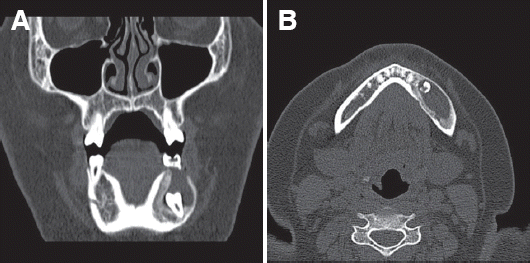 | Fig. 4CT view after 6 months follow-up. Perforation of mandible is not detected. (A) Coronal view, (B) Axial view. 
|
 | Fig. 5CT view after 8 months follow-up. Marked newly formed bone is detected. (A) Coronal view, (B) Axial view. 
|
 | Fig. 6The inferior alveolar nerve is protected with newly formed bone (arrow: inferior alveolar nerve). 
|
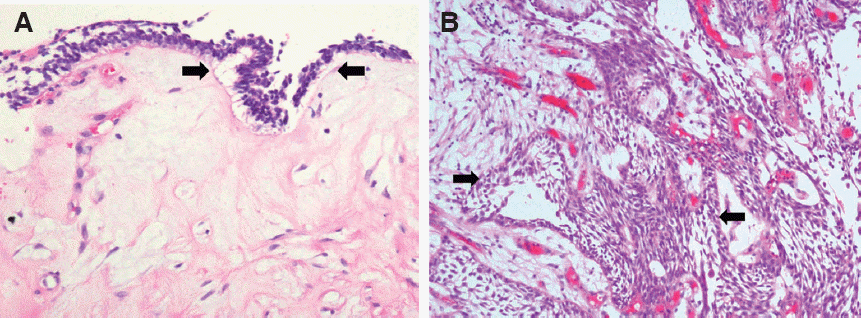 | Fig. 7Microscopically, unilocular cyst is lined by thin epithelium with ameloblastic changes such as basaloid 
|
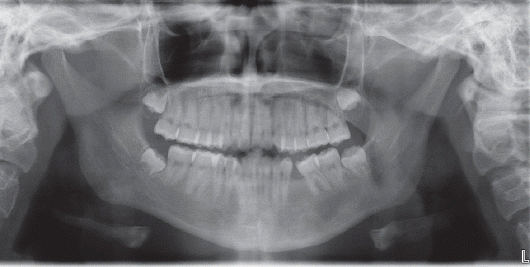 | Fig. 8Dental panoramic view after 2 years follow-up. Completely healed mandibular lesion is detected. 
|
appearance, palisading nuclei and subnuclear clearing (arrows). But it is confined to the cystic lining and shows a very thin lining (×200), (B) This unicystic tumor shows classic histologic features of ameloblastoma, palisading of the nuclei with reverse polarity and nuclear clearing is seen (arrows) (×400).
Go to :

Discussion
UA, which was first described by Robinson & Martinez in 1977, has a unilocular feature in radiologic examination and a cystic feature histologically.
1,
3 The treatment options of UA are radical (resection and reconstruction using a reconstruction plate) or conservative (enucleation, enucleation after application of Carnoy’s solution, or enucleation after marsupialization).
1 The outcome of UA is better after conservative treatment compared to other types of ameloblastoma (solid and multicystic).
7
In case of UA extending to the mandibular condyle or inferior alveolar nerve, radical resection might result in facial deformity and nerve damage, which directly influences quality of life.
9-
11
UA was classified into 3 subtypes by Ackermann histologically: subtype 1 is unilocular cystic luminal; subtype 2 is intraluminal with a solid growth inside
lumen of the cystic lesion; and subtype 3 is intraluminal growth with mural invasion within adjacent tissues.
2 Subtype 1 and 2 shows better treatment response to conservative treatments such as marsupialization and enucleation. On the other hand, subtype 3 is treated with more aggressive treatment options such as resection.
12 Therefore, it might be better that an incisional biopsy should be performed to determine the histopathologic subtype, for considering of treatment plan.
7
The rationale for conservative therapy is decompression of the mass using marsupialization.
13 Mass decompression promotes bone remodeling and therefore osteogenesis.
11,
13 In our case, UA invaded the inferior alveolar nerve. Therefore, radical treatment would result in severe nerve damage. However, the use of decompression and marsupialization allowed to successfully performing nerve protection followed by osteogenesis around the inferior alveolar nerve 8 months after procedure. Hence, after surgery the patient had no nerve damage or facial deformity.
Because of the characteristics of UA, which is similar to a non-neoplastic odontogenic cyst, it can be misdiagnosed as an odontogenic keratocyst, dentigerous cyst, or other odontogenic cysts. Therefore, histological differential diagnosis is essential.
9
In conclusion, because the resection of extensive UA might impair swallowing, mastication, and speech due to dentofacial deformity, especially in children,
12 we recommend a conservative approach in children due to their development of the craniofacial region. Additional efforts for reducing recurrence rate will be needed.
Go to :








 PDF
PDF Citation
Citation Print
Print







 XML Download
XML Download