1. Claffey N, Egelberg J. Clinical indicators of probing attachment loss following initial periodontal treatment in advanced periodontitis patients. J Clin Periodontol. 1995; 22:690–6. DOI:
10.1111/j.1600-051X.1995.tb00828.x. PMID:
7593699.
2. Matuliene G, Pjetursson BE, Salvi GE, Schmidlin K, Brägger U, Zwahlen M, Lang NP. Influence of residual pockets on progression of periodontitis and tooth loss: results after 11 years of maintenance. J Clin Periodontol. 2008; 35:685–95. DOI:
10.1111/j.1600-051X.2008.01245.x. PMID:
18549447.
5. Cortellini P, Labriola A, Tonetti MS. Regenerative periodontal therapy in intrabony defects: state of the art. Minerva Stomatol. 2007; 56:519–39. PMID:
18091668.
6. Cortellini P, Tonetti MS. A minimally invasive surgical technique with an enamel matrix derivative in the regenerative treatment of intra-bony defects: a novel approach to limit morbidity. J Clin Periodontol. 2007; 34:87–93. DOI:
10.1111/j.1600-051X.2007.01144.x. PMID:
17243998.
7. Sculean A, Nikolidakis D, Nikou G, Ivanovic A, Chapple IL, Stavropoulos A. Biomaterials for promoting periodontal regeneration in human intrabony defects: a systematic review. Periodontol 2000. 2015; 68:182–216. DOI:
10.1111/prd.12086. PMID:
25867987.
8. Esposito M, Grusovin MG, Papanikolaou N, Coulthard P, Worthington HV. Enamel matrix derivative (Emdogain) for periodontal tissue regeneration in intrabony defects. A Cochrane systematic review. Eur J Oral Implantol. 2009; 2:247–66. PMID:
20467602.
9. Kao RT, Nares S, Reynolds MA. Periodontal regeneration - intrabony defects: a systematic review from the AAP Regeneration Workshop. J Periodontol. 2015; 86:S77–104. DOI:
10.1902/jop.2015.130685. PMID:
25216204.
10. Lekovic V, Camargo PM, Weinlaender M, Nedic M, Aleksic Z, Kenney EB. A comparison between enamel matrix proteins used alone or in combination with bovine porous bone mineral in the treatment of intrabony periodontal defects in humans. J Periodontol. 2000; 71:1110–6. DOI:
10.1902/jop.2000.71.7.1110. PMID:
10960017.
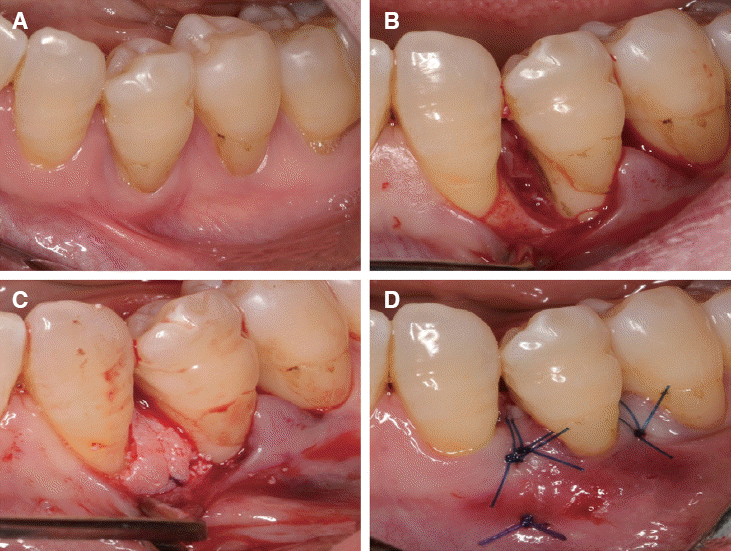
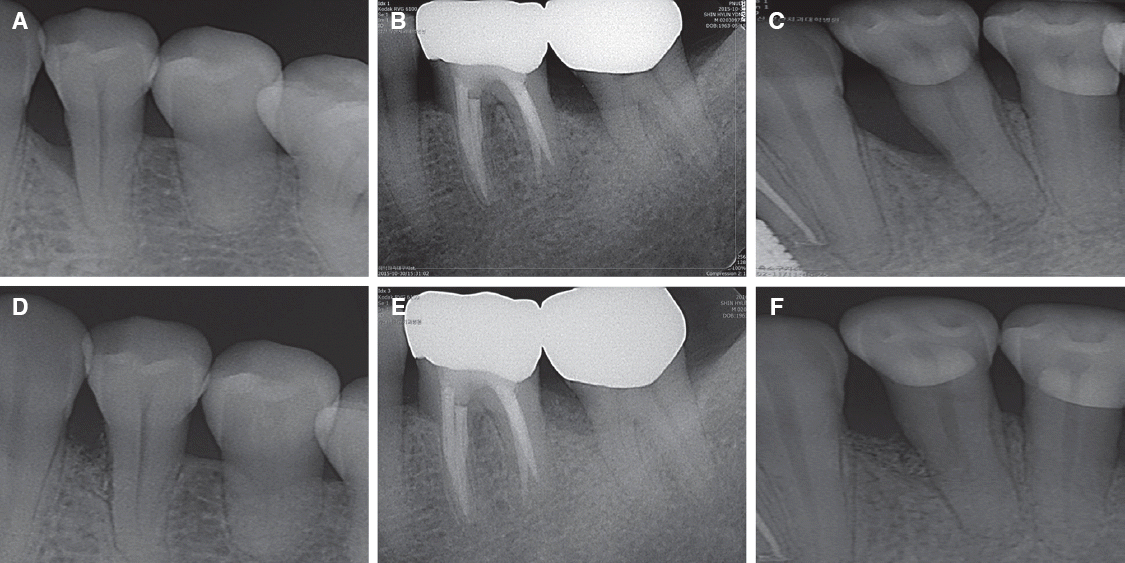
11. Cortellini P, Prato GP, Tonetti MS. The simplified papilla preservation flap. A novel surgical approach for the management of soft tissues in regenerative procedures. Int J Periodontics Restorative Dent. 1999; 19:589–99. PMID:
10815597.
12. Saimbi CS, Gautam A, Khan MA, Nandlal . Periosteum as a barrier membrane in the treatment of intrabony defect: a new technique. J Indian Soc Periodontol. 2014; 18:331–5. DOI:
10.4103/0972-124X.134571. PMID:
25024547. PMCID:
PMC4095626.
13. Lekovic V, Kenney EB, Carranza FA, Martignoni M. The use of autogenous periosteal grafts as barriers for the treatment of Class II furcation involvements in lower molars. J Periodontol. 1991; 62:775–80. DOI:
10.1902/jop.1991.62.12.775. PMID:
1765940.
14. Trombelli L, Simonelli A, Pramstraller M, Wikesjö UM, Farina R. Single flap approach with and without guided tissue regeneration and a hydroxyapatite biomaterial in the management of intraosseous periodontal defects. J Periodontol. 2010; 81:1256–63. DOI:
10.1902/jop.2010.100113. PMID:
20528696.
15. Rakmanee T, Griffiths GS, Auplish G, Darbar U, Petrie A, Olsen I, Donos N. Treatment of intrabony defects with guided tissue regeneration in aggressive periodontitis: clinical outcomes at 6 and 12 months. Clin Oral Investig. 2016; 20:1217–25. DOI:
10.1007/s00784-015-1608-z. DOI:
10.1007/s00784-015-1609-y.
16. Machtei EE. The effect of membrane exposure on the outcome of regenerative procedures in humans: a meta-analysis. J Periodontol. 2001; 72:512–6. DOI:
10.1902/jop.2001.72.4.512. PMID:
11338304.
17. Kwan SK, Lekovic V, Camargo PM, Klokkevold PR, Kenney EB, Nedic M, Dimitrijevic B. The use of autogenous periosteal grafts as barriers for the treatment of intrabony defects in humans. J Periodontol. 1998; 69:1203–9. DOI:
10.1902/jop.1998.69.11.1203. PMID:
9848529.
18. Gamal AY, Mailhot JM. A novel marginal periosteal pedicle graft as an autogenous guided tissue membrane for the treatment of intrabony periodontal defects. J Int Acad Periodontol. 2008; 10:106–17. PMID:
19055224.
19. Tonetti MS, Prato GP, Cortellini P. Factors affecting the healing response of intrabony defects following guided tissue regeneration and access flap surgery. J Clin Periodontol. 1996; 23:548–56. DOI:
10.1111/j.1600-051X.1996.tb01823.x. PMID:
8811474.