|
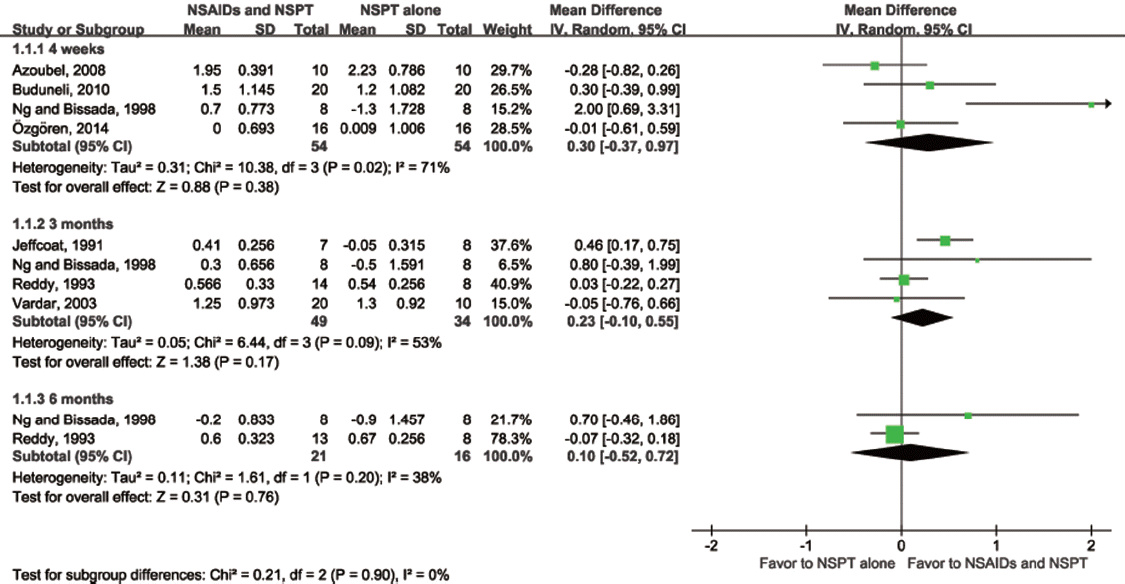
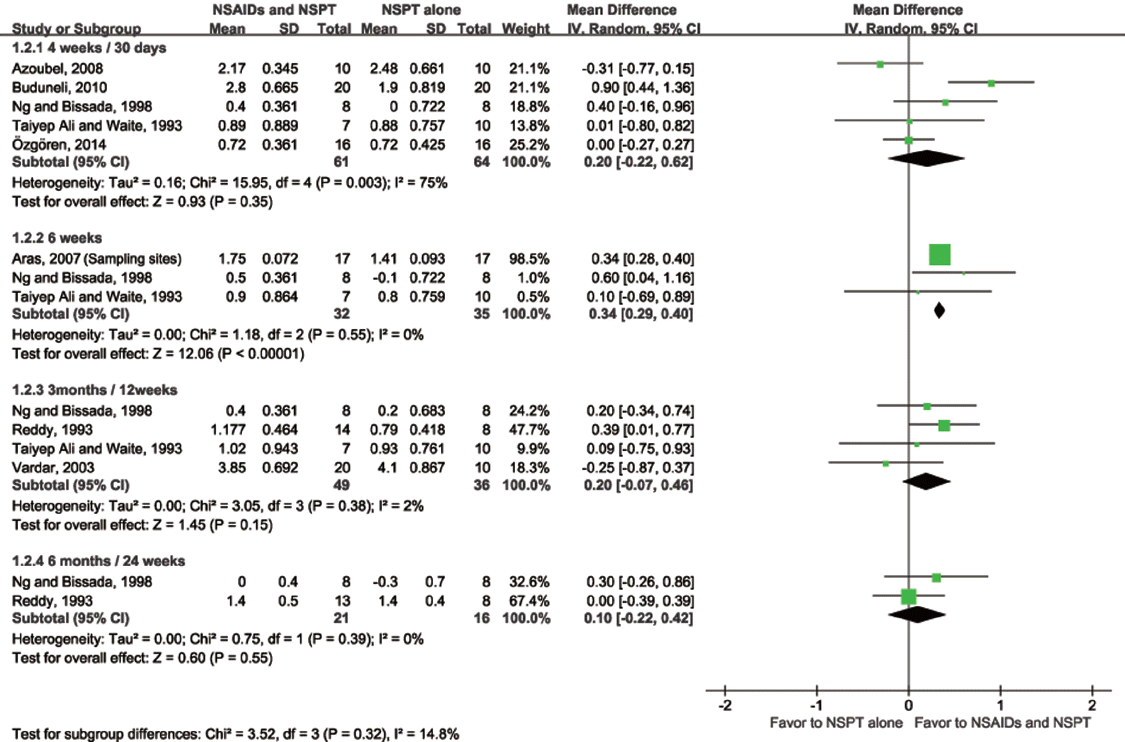
Aras 2007 |
RCT
Sample size at entry: naproxen: 17, placebo: 17 Study duration: 6 weeks Unit of observation: subject |
Age (mean ± SD, yrs): 36.24 ± 2.4
Sex % F: 50%, not mentioned in each group
Smoker: excluded
DM: excluded |
Rx) naproxen 275 mg 1T for 6 weeks Ctrl) placebo for 6 weeks Com) supragingival scaling, OHI at baseline |
Subject based: mean ± SD of PD, GI, PI, gingival bleeding index at baseline and 6 weeks (full mouth) mean ± SD of PD, GI, PI, gingival bleeding index at baseline and 6 weeks (sample site) |
Probing site: full mouth Sampling sites: two deepest periodontal pockets in the maxilla incisors, canines, and premolar
No. of probing sites: not mentioned |
|
Azoubel 2008 |
RCT
Sample size at entry: etoricoxib: 11, placebo: 10 Study duration: 30 days
Unit of observation: subject, site |
Patient: AP, age of 18 – 35 years, had ≥ 20 teeth, had ≥ 4 sites in different teeth showing PD of ≥ 4 mm, and had ≥ 2 sites showing PD of ≥ 7 mm Age (mean ± SD, yrs): etoricoxib: 32.4 ± 6.5, placebo: 34.6 ± 7.6 Sex % F: etoricoxib: 91%, placebo: 90% Smoker: excluded
DM: excluded |
Rx) etoricoxib 120 mg 1T for 7 days Ctrl) placebo 1T for 7 days Com) OHI and SRP during drug intake, weekly oral hygiene reinforcement and prophylaxis until 30 days |
Subject-based: mean ±SD of PD, CAL, gingival recession, visual plaque index, and BOP at baseline and 30 days Site-based: frequency (%) of sites showing CAL gain of ≥ 2 mm Dropout and reason: Rx) 1, lost to followup (did not return for reassessment) |
Probing site: sites showing baseline PD of >3 mm only and gingival increase was excluded Sampling sites: deepest periodontal pockets in the maxilla anterior and premolar No. of probing sites: 6 |
|
Buduneli 2010 |
RCT
Sample size at entry: meloxicam: 26, placebo: 24
Study duration: 4 weeks
Experimental unit: subject |
Patient: CP, Caucasian, had ≥ 4 sites showing CAL of ≥ 4 mm and PD of ≥ 5 mm and two of which were in the anterior region
Age (mean ± SD, yrs): meloxicam: 48.4 ± 2.1, placebo: 47.2 ± 1.9
Sex % F: meloxicam 50%, placebo 50% Smoking: excluded
DM: excluded |
Rx) meloxicam 7.5 mg 1T for 10 days Ctrl) placebo for 10
Days Com) SRP on day 3 of drug intake |
Subject-based: mean ± SD of PD, CAL, dichotomous plaque index (present or absent), and papilla bleeding index (PBI) at 10 days and 4 weeks after SRP
Dropout and reason: Rx) 3, Ctrl) 2, discontinued
study |
Probing, sampling sites: 2 sampling sites of the single-root teeth showing PD of ≥ 5 mm, CAL of ≥ 4 mm, and BOP (+) No. of probing sites per tooth: 2 (mesiobuccal, distobuccal) |
|
Jeffcoat 1991 |
RCT
Sample size at entry: naproxen: 7, placebo: 8
Study duration: 3 months
Unit of observation: subject |
Patient: AP, had ≥ 5 sites showing radiographic evidence of rapidly progressive periodontitis, age < 35, and had ≥ 20 teeth
Age (range, yrs): 18 - 41 Sex F %: overall 73%, no detail per group
Smoking: not mentioned
DM: excluded |
Rx) naproxen 1000 mg per day for 3 months
Ctrl) placebo for 3 months
Com) SRP 2 weeks before drug intake, OHI 2 weeks before drug intake and after 1, 2, 3 months of drug intake |
Subject-based: mean ± SD of the change in CAL and GI between baseline and 3 months
Site-based: radiographic: frequency (%) of tooth showing increased, no change, or decreased bone |
No. probing sites: five test sites which had evidence of rapidly progressive periodontitis |
|
Ng and Bissada 1998 |
RCT Split mouth design (SRP / no SRP) Sample size at entry: no detail (32 subjects were randomly assigned to one of the 4 treatment group)
Study duration: 24 weeks Unit of observation: subject |
32 subjects with generalized periodontitis that radiographic bone loss is greater than 50% in at least 2 matching tooth types per subject, PD of ≥ 5 mm in 1 or more sites in at least 2 matching types Age (range, yrs): 32 - 72 yrs Sex F %: overall 44%, no detail per group
Smoking: smoker = 15, non-smoker + former smoker = 17
DM: excluded |
Rx1) doxycycline 200 mg (first day), 100 mg (other day) per day for 6 weeks
Rx2) ibuprofen 800 mg per day for 6 weeks
Rx3) doxycycline + ibuprofen for 6 weeks Ctrl) placebo for 6 weeks
Com) SRP at baseline |
Subejct-based: mean ± SD of PI, GI, PD, CAL at baseline, 3, 6, 9, 12, and 24 weeks |
Probing sites: full mouth
No. of probing sites per tooth: 6 (using pre-fabricated stents) |
|
Özgören 2014 |
RCT
Sample size at entry: tenoxicam: 16, placebo: 16
Study duration: 30 days
Unit of observation: subject |
Patient: CP, had ≥ 4 periodontal sites showing PD of 4 - 6 mm and radiographic evidence of bone and attachment loss involving the maxillary anterior teeth
Age (mean ± SD yrs): tenoxicam: 40.9 ± 8.2, placebo: 42.3 ± 7.3
Sex F %: overall 44%, no details per group
Smoking: excluded
DM: excluded |
Rx) tenoxicam 20 mg
per day for 10 days
Ctrl) placebo for 10 days
Com) SRP at baseline,
OHI before baseline
and at baseline |
Subject-based: mean ± SD of PI, GI, PD, gingival bleeding time index and CAL at baseline and 30 days |
Probing sites: 4 teeth (PD of 4 - 6 mm and radiographic evidence of bone and attachment loss involving the maxillary anterior teeth) No. of probing sites per tooth: 6 |
|
Pinho Mde 2008 |
RCT
Sample size at entry: loxoprofen 30, placebo: 30
Study duration: 28 days
Unit of observation: subject |
Patient: periodontitis, had ≥ 20 teeth, 35 - 0 years, had ≥ 2 teeth showing CAL of ≥ 6 mm, and had ≥ 1 site showing PD of ≥ 5 mm
Age (mean ± SD): loxoprofen 41 ± 4, placebo: 42 ± 5
Sex F%: loxoprofen 53%, placebo: 53%
Smoking: excluded
DM: excluded |
Rx) loxoprofen 60 mg per day for 28 days
Ctrl) placebo for 28
days
Com) SRP of half mouth at baseline and contra-lateral side on
day 14 and OHI at baseline and 14 days |
Subject-based: PD, % BOP, and PI at baseline (demographic data) Site-based: % sites showing PD of < 4, 4 - 7, and ≥ 7 mm at baseline,
14, and 28 days |
Probing sites: full mouth
No. of probing sites per tooth: 6 |
|
Reddy 1993 |
RCT Sample size at entry: overall 27, no details per group
Study duration: 28 days
Unit of observation: subject, site |
Patient: had a history of periodontitis prior to the age of 35, had ≥ 3 posterior teeth in each of 3 quadrants showing alveolar bone loss of 30 - 50%, and had ≥ 3 sites showing active bone loss as determined by bone scan
Age (mean ± SD, yrs): overall: 36.5 ± 7.88, no details per group
Sex F %: overall 68%
Smoking: not mentioned
DM: excluded |
Rx1) meclofenamate 100 mg per day for 6 months
Rx2) meclofenamate 200 mg per day for 6 months
Ctrl) placebo for 6 months
Com) OHI, SRP, and occlusal adjustment (when needed) before 2 - 4 weeks from baseline SRP at 3 months and 6 months |
Subject-based: GI, PI, PD and CAL at baseline, 3 months, and 6 months Site-based: CAL at baseline to 6 months
Dropout and reason: Rx1) 3 (2: gastrointestinal irritation, 1: disliked medication)
Rx2) 1 (disliked medication)
Ctrl) 1 (military service) Adverse effect gastrointestinal irritation |
Probing sites: 6 - 8 teeth (which show high bone-seeking radiopharmaceutical uptake ratio)
No. of probing sites per tooth: not mentioned Statistics were expressed as mean ± standard error |
|
Shiloah 2014 |
RCT
Sample size at entry: aspirin: 18, placebo 14
Study duration: 12 months
Unit of observation: subject |
Patient: chronic periodontitis, self-reported smoker, had ≥ 4 teeth showing PD of 5 mm and attachment loss of > 2 mm
Age (mean ± SD yrs): aspirin: 53.25 ± 7.31, placebo: 48.83 ± 8.34
Sex F %: 58% except dropout, not mentioned at entry
Smoking: 100% (only smoker included)
DM: excluded |
Rx) aspirin 325 mg per day for a year
Ctrl) placebo for a year
Com) extraction of “hopeless”, non-restorable tooth, OHI, SRP before baseline |
Subject-based:
% sites plaque index = 2 and 3, % sites GI = 0, 1, and 2, % sites PD 1 - 3, 4 - 6, and ≥ 7 mm, % sites PAL 0 - 2, 3 - 4, and > 4 mm, % sites BOP at baseline, 3, 6, 9, and 12 months Dropout and reason
Rx) 6 (lost to follow up, e.g., job schedule change, moved)
Ctrl) 2 (lost to follow up e.g., job schedule change, moved) |
Probing sites: full mouth
No. of probing sites per tooth: not mentioned |
|
Taiyep Ali and Waite 1993 |
RCT Sample size at entry: overall 17, no details per group
Study duration: 8
Weeks Unit of observation: subject |
Patient: chronic periodontitis, had 6 Ramfjord teeth with mild to severe bone loss Age (range yrs): overall 28 - 40
Sex F %: overall: 71%, no details per group
Smking: not mentioned
DM: excluded |
Rx) ibuprofen 800 mg per day for 14 days
Ctrl) no medication
Com) OHI, SRP (split mouth) at baseline;
OHI, oral prophylaxis, and re-evaluation at 2, 4, 6 and 8 weeks |
Subject-based: PD, gingival color index, and gingival bleeding at 2, 4, 6, and 8 weeks
Dropout and reason: Rx) 2, unknown |
Probing sites: 6 teeth (Ramfjord teeth)
No. of probing sites per tooth: 6 No placebo medication |
|
Vardar 2003 |
RCT Sample size at entry: nimesulide 10, naproxen 10, and placebo 10 Study duration: 3 months
Unit of observation: subject |
Patient: CP, had ≥ 18 teeth, and had ≥ 2 teeth in each quadrant showing PD of ≥ 5 mm and CAL of ≥ 4 mm
Age (mean ± SD, yrs): nimesulide: 46.6 ± 14.1, naproxen: 44.0 ± 3.6, placebo: 43.9 ± 6.9 Sex F %: nimesulide: 40%, naproxen: 30%, placebo: 50%
Smoker: not excluded
DM: excluded |
Rx1) nimesulide 200 mg per day for 10 days
Rx2) naproxen 550 mg per day for 10 days
Ctrl) placebo for 10 days
Com) OHI at baseline, SRP at 3 days |
Subejct-based: CAL and PD at baseline and 3 months
PI, PBI at baseline, 10 days, and 3 months |
Probing sites: 4 sites (2 sampling sites x 2 approximal tooth sites adjacent to the sampling site) |
|
Yen 2008 |
RCT Sample size at entry: overall 131
Study duration: 12 months
Unit of observation: site |
Patient: CP, had > 16 teeth (at least two of which were molars), had ≥ 4 teeth showing PD of > 4 mm and CAL of > 2 mm, and had ≥ 2 interproximal areas showing radiographic evidence of bone loss
Age (mean ± SD, yrs): overall 48.6 ± 9.9
Sex F%: 47% at 3 months Smoker: 34.7% (former smoker: 23.8%, nonsmoker: 39.6%)
DM: not excluded |
Rx) celecoxib 200 mg per day for 3 months
Ctrl) placebo for 3 months
Com) SRP at baseline (1/2) and within 1 - 2 weeks (1/2)
OHI at baseline, 3, 6, 9, and 12 months oral prophylaxis at 3, 6, 9, and 12 months |
Site-based CAL by BPD = 1 - 3, 4 - 6, and ≥ 7 mm PD by BPD = 1 - 3, 4 - 6, and ≥ 7 mm % sites showing CAL gain ≥ 2 mm % sites showing CAL loss ≥ 2 mm
Dropout and reason Total 66 (highly publicized finding that subjects taking large dosages of COX-2 inhibitors showed an increased risk for
cardiovascular episodes) |
Probing sites: full mouth No. of probing sites per tooth: 6 66 subjects were dropout |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download