Introduction
Materials and Methods
Data collection
Table 1
Gender
Age at implant placement (three groups: < 45 years, 45 - 54 years, and ≥ 55 years)
History of type 2 diabetes mellitus; smoking status (a smoker was classified as at least 1 cigarette per day at the time of implant placement)
Surface of implant (three groups: blasted, anodized, and blasted and acid etched surface); implant product used according to the abutment connection type [two groups: internal type (1. Implantium, Dentium, Seoul, Korea; 2. GS II, Osstem, Seoul, Korea; 3. Osseospeed, Astra Tech AB, Mölndal, Sweden; 4. Replace, Nobel Biocare AB, Gothenburg, Sweden; and external type (5. Biomet 3i osseotite, Implant Innovations, Palm Beach Gardens, FL, USA; 6. Brånemark Mk III, Nobel Biocare AB; 7. Neoplant, Neobiotech, Seoul, Korea; 8. US II, Osstem)]
Implant diameter (three groups: < 4 mm, 4 - 4.5 mm, and ≥ 5 mm)
Location of implant within the dental arch (four groups: maxillary anterior, maxillary posterior, mandibular anterior, and mandibular posterior regions)
Year of the resident at the time of implant placement
Spontaneous early exposure of cover screw (CS) during the healing phase
Prosthetic complications (fracture of abutment, abutment screw and implant prosthesis; loosening of abutment screw and implant prosthesis; and chipping of veneering ceramic)
Bone augmentation procedures [procedures such as guided bone regeneration (GBR), sinus elevation with crestal approach (Crestal), and sinus elevation with lateral approach (Lateral). If GBR and one of the sinus elevation procedures were applied to the same area simultaneously, the implant was classified as sinus elevation group]
Peri-implant plastic surgeries (procedures such as apically repositioned flap and gingival graft)
Dates of implant placement, prosthesis placement, implant removal, and the last follow-up visit.
Peri-implant marginal bone level evaluation
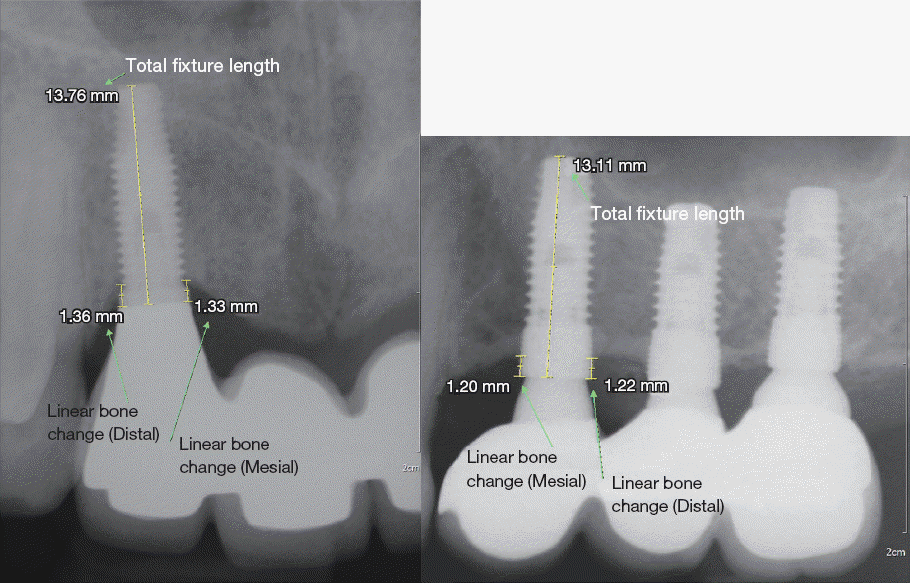 | Fig. 2Radiographs of the measurements of linear bone change and total fixture length. MBL was assessed by measuring the linear bone change from the abutment-fixture junction to the first bone-to-implant contact at the mesial and distal site of each implant. After comparing the mesial and distal bone loss values, the higher of the two was considered the MBL. |
Statistical analyses
Results
Demographic characteristics
Table 2
| Survived implants | Failed implants | Total implants | P value | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Gender | 0.023* | |||
| Female | 147 (35.0) | 2 (0.5) | 149 (35.5) | |
| Male | 252 (60.0) | 19 (4.5) | 271 (64.5) | |
| Age (years) | 0.833 | |||
| < 45 | 108 (25.7) | 5 (1.2) | 113 (26.9) | |
| 45 - 54 | 201 (47.9) | 12 (2.9) | 213 (50.7) | |
| ≥ 55 | 90 (21.4) | 4 (1.0) | 94 (22.4) | |
| Mean (SD) | 49.3 (6.72) | 48.9 (6.30) | 49.3 (6.69) | |
| Type 2 DM | 0.550 | |||
| No | 364 (86.9) | 20 (4.8) | 384 (91.7) | |
| Yes | 34 (8.1) | 1 (0.2) | 35 (8.3) | |
| Smoking | 0.002** | |||
| No | 286 (68.1) | 8 (1.9) | 294 (70.0) | |
| Yes | 113 (26.9) | 13 (3.1) | 126 (30.0) | |
Implant characteristics
Table 3
| Survived implants | Failed implants | Total Implants | P value | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Abutment connection | 0.490 | |||
| Internal | 103 (24.5) | 4 (1.0) | 107 (25.5) | |
| External | 296 (70.5) | 17 (4.0) | 313 (74.5) | |
| Surface of implant | 0.611 | |||
| Blasted | 165 (39.3) | 11 (2.6) | 176 (41.9) | |
| Anodized | 96 (22.9) | 4 (1.0) | 100 (23.9) | |
| Blasted and acid etched | 138 (32.9) | 6 (1.4) | 144 (34.3) | |
| Diameter (mm) | 0.596 | |||
| < 4 | 54 (12.9) | 0 (0.0) | 54 (12.9) | |
| 4 to 4.5 | 264 (62.9) | 14 (3.3) | 278 (66.2) | |
| ≥ 5 | 81 (19.3) | 7 (1.7) | 88 (21.0) | |
| Mean (SD) | 4.21 (0.23) | 4.46 (0.12) | 4.22 (0.46) | |
| Implant location | 0.028* | |||
| Mandibular anterior | 13 (3.1) | 0 (0.0) | 13 (3.1) | |
| Maxillary anterior | 32 (7.6) | 0 (0.0) | 32 (7.6) | |
| Mandibular posterior | 200 (47.6) | 4 (1.0) | 204 (48.6) | |
| Maxillary posterior | 154 (36.7) | 17 (4.0) | 181 (40.7) | |
Surgical characteristics and the presence of complications
Table 4
| Survived implants | Failed implants | Total implants | P value | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Resident experience | 0.171 | |||
| 1st year | 12 (2.9) | 1 (0.2) | 13 (3.1) | |
| 2nd year | 219 (52.1) | 7 (1.7) | 226 (53.8) | |
| 3rd year | 168 (40.0) | 13 (3.1) | 181 (43.1) | |
| Spontaneous CS exposure | < 0.001** | |||
| Absence | 342 (81.4) | 10 (2.4) | 352 (83.8) | |
| Presence | 57 (13.6) | 11 (2.6) | 68 (16.2) | |
| Prosthetic complications | 0.561 | |||
| Absence | 358 (85.2) | 18 (4.3) | 376 (89.5) | |
| Presence | 41 (9.8) | 3 (0.7) | 44 (10.5) | |
| Bone augmentation surgery | 0.001** | |||
| Conventional | 224 (53.3) | 8 (1.9) | 232 (55.2) | |
| GBR | 107 (25.5) | 2 (0.5) | 109 (26.0) | |
| S/E (Crestal) | 30 (7.1) | 7 (1.7) | 37 (8.8) | |
| S/E (Lateral) | 38 (9.0) | 4 (1.0) | 42 (10.0) | |
| Peri-implant plastic surgery | 0.417 | |||
| Absence | 270 (64.3) | 16 (3.8) | 286 (68.1) | |
| Presence | 129 (30.7) | 5 (1.2) | 134 (31.9) | |
Implant survival
Table 5
Risk factors associated with implant survival
Table 6
| Multivariate Odds ratio | 95% Confidence Interval | P value | |
|---|---|---|---|
| Smoking | 0.033* | ||
| Smoking | 3.263 | 1.097 to 9.707 | 0.033* |
| Implant position | 0.530 | ||
| Mandibular posterior | 0.358 | 0.092 to 1.388 | 0.137 |
| Maxillary anterior | < 0.001 | 0.000 | 0.998 |
| Mandibular anterior | < 0.001 | 0.000 | 0.999 |
| Spontaneous CS exposure | < 0.001** | ||
| Presence | 6.523 | 2.411 to 17.650 | < 0.001** |
| Bone augmentation procedure | 0.206 | ||
| Guided bone regeneration | 0.493 | 0.092 to 2.645 | 0.409 |
| Sinus elevation (Crestal) | 2.896 | 0.766 to 10.953 | 0.117 |
| Sinus elevation (Lateral) | 1.034 | 0.228 to 4.695 | 0.965 |
Peri-implant marginal bone level
Table 7
| Variables | β ± SE | P value |
|---|---|---|
| Smoking | 0.505 ± 0.131 | < 0.td** |
| Patient gender | -0.051 ± 0.126 | 0.687 |
| Patient age | 0.009 ± 0.009 | 0.316 |
| Resident experience | -0.126 ± 0.111 | 0.254 |
| Resident experience (2 - 3) | -0.256 ± 0.124 | 0.040* |
| Abutment connection | 0.837 ± 0.132 | < 0.001** |
| Implant product | 0.135 ± 0.026 | < 0.001** |
| Diameter of implant | -0.323 ± 0.132 | 0.015* |
| Implant position | -0.025 ± 0.045 | 0.583 |
| Cover screw exposure | 0.236 ± 0.172 | 0.169 |
| Prosthetic complication | 0.045 ± 0.200 | 0.821 |
| Bone augmentation surgery | 0.027 ± 0.063 | 0.663 |
| Periodontal plastic surgery | -0.035 ± 0.129 | 0.787 |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download