Introduction
Materials and Methods
Fungal species and cultivation
Production of various electrolyzed water
Antifungal activity of the electrolyzed water against C. albicans
Preparation of plate for candidal biofilm
Antifungal activity of the electrolyzed water against C. albicans biofilm
Comparison of antifungal activity of various electrolyzed water against C. albicans biofilm
Statistical analysis
Results
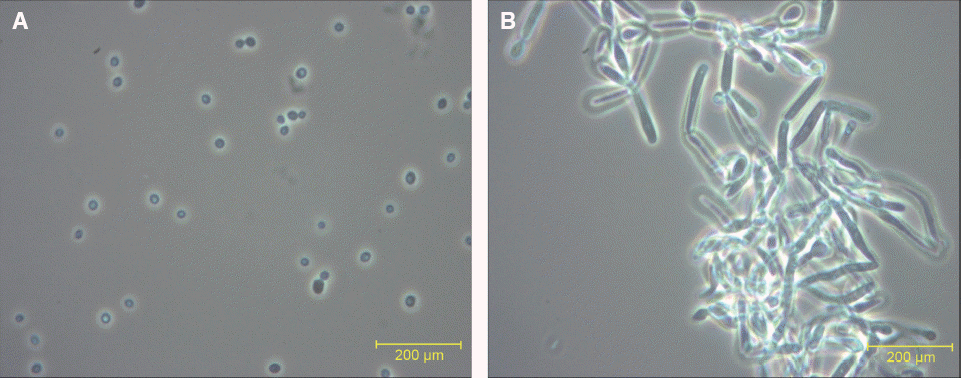
Investigation of culture condition for blastoconidia and hyphae of C. albicans
Antifungal activity of the electrolyzed water against C. albicans
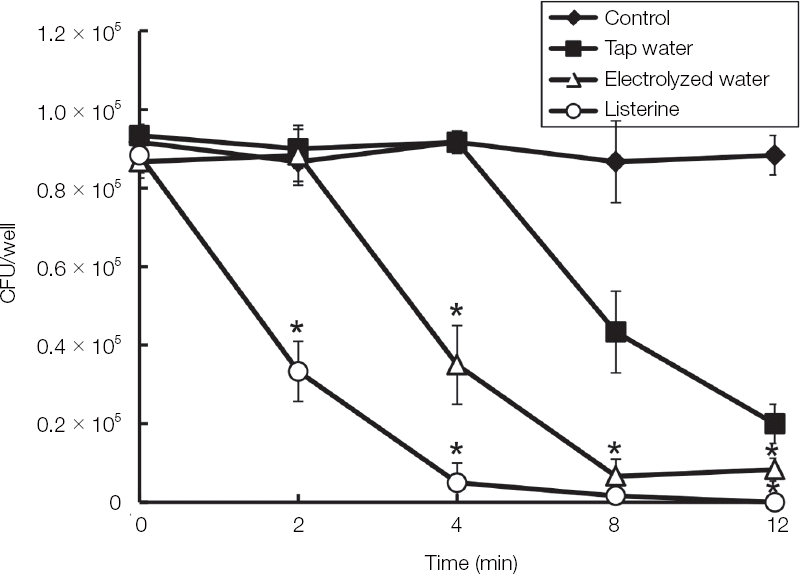 | Fig. 2The antifungal activity of the EWP against blastoconidia type of C. albicans. C. albicans was cultivated by using sabouraud dextrose broth in aerobic condition overnight and then harvested by centrifugation. The pellet of C. albicans was treated with tap water, EWP and listerine in various times. The live C. albicans was counted by colonies after plating on sabouraud dextrose agar. The examinations were performed three times. * Indicates a significant difference compared with control (P < 0.05). |
Antifungal activity of the electrolyzed water against C. albicans biofilm
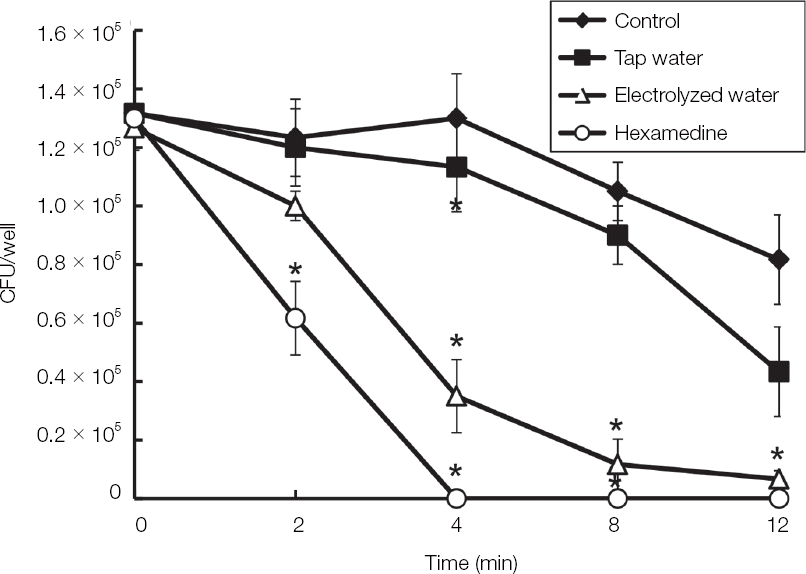 | Fig. 3The antifungal activity of the EWP against C. albicans biofilm. C. albicans was cultivated by using F-12 media in 5% CO2 condition overnight and then harvested by centrifugation. The pellet of C. albicans was treated with tap water, EWP and listerine in various times. The live C. albicans was counted by colonies after plating on sabouraud dextrose agar. The examinations were performed three times. * Indicates a significant difference compared with control (P < 0.05). |
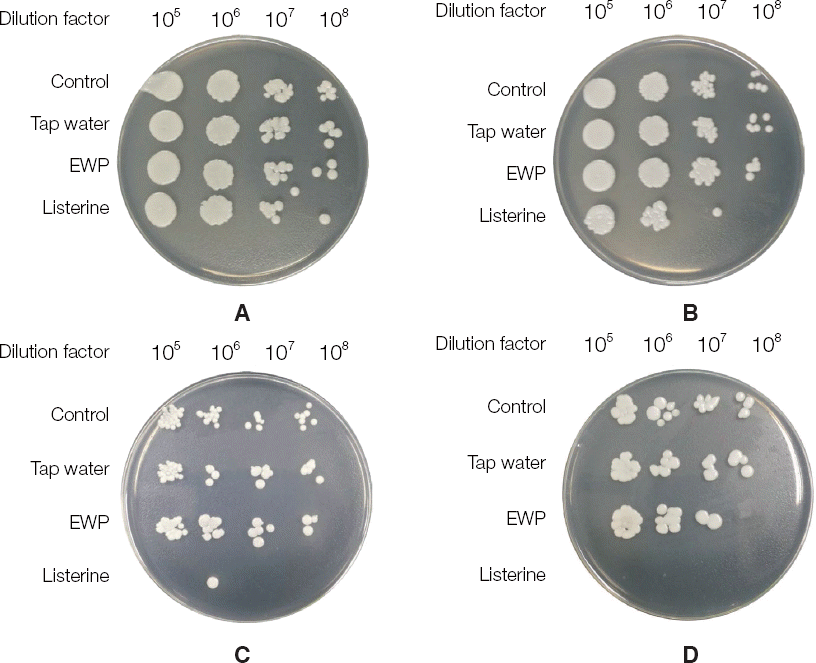 | Fig. 4The image of antifungal activity against C. albicans biofilm. C. albicans was cultivated using F-12 media in 5% CO2 condition overnight and then harvested by centrifugation. The pellet of C. albicans was treated with tap water, electrolyzed water and listerine in various times and then inoculated on sabouraud dextrose agar. (A) 2 minutes, (B) 4 minutes, (C) 8 minutes, (D) 12 minutes. EWP, electrolyzed water using palladium electrode. |
Comparison of antifungal activity of various electrolyzed water against C. albicans biofilm
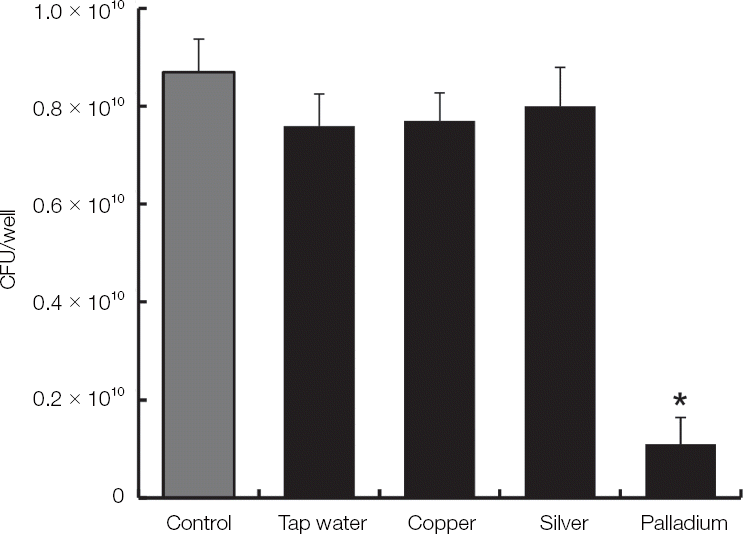 | Fig. 5The antifungal activity of the electrolyzed water using various electrodes against C. albicans. C. albicans was cultivated by sabouraud dextrose broth in aerobic condition overnight and then harvested by centrifugation. The pellet of C. albicans was treated with tap water and electrolyzed water using various electrodes for 12 minutes. The live C. albicans was counted by colonies after plating on sabouraud dextrose agar. The examinations were performed three times. * Indicates a significant difference compared with control (P < 0.05). |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download