Introduction
Endosseous dental implants have been utilized as successful rehabilitative therapy in restoring partially and completely edentulous arches since osseointegration theory was introduced. Recently, the success rates of implants have increased markedly with the help of thorough understanding about bony responses and loading concepts. However, it may be difficult to determine the exact mechanism leading to implant failure because multiple factors contribute to the failure of implants indirectly as well as directly.
1
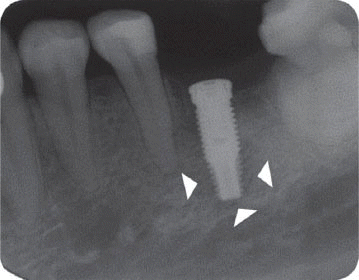
Among those factors, the main causes of implant failure can be peri-implantitis and overloading to implants. It has been reported that the implant periapicallesion (IPL) also could be one of the complications leading to implant failure though its prevalence would be quite low. IPL is defined as a radiolucent lesion around the apical part of a stable implant,
2 which is characterized by suppuration, fistula formation, and loss of alveolar bone.
3 It is often found at the apical part of the long implant located at the cortical bone being stably supported and intimately contacted by normal bone at coronal part of it.
4 It is also called retrograde peri-implantitis related to premature loading, overloading, and microfracture by other trauma or occlusal factors.
5 The infection around apical part of implant can spread out coronally, proximally, and buccolingually. It can eventually destruct the alveolar bone near the infected implant devitalizing the adjacent teeth and compromising stability of implant.
6
The purpose of this report is to suggest clinical managements of IPLs by presenting three clinical cases managed by either the infected form or the inactive form with the follow-up period of five to twelve years.
Go to :

Discussion
The incidence of IPL is quite low. Reiser and Nevins
7 have reported that the incidence of IPL was 0.26% (10 out of 3,800) and had occurred more in maxilla than in mandible, especially in maxillary premolar areas.
8 Stephen et al.
3 have reported that the incidence was 9.9% (39 out of 395) and symptomatic lesions were 66.7%. Among those who had implant therapy in department of periodontics, Gangneung-Wonju National University Dental Hospital, the incidence of IPL was 0.66% (15 out of 2,289) and occurred six times more in mandible than in maxilla.
IPL is divided into the inactive form and the infected form according to the clinical symptoms.
7 The inactive form may be similar to the periapical scar without any clinical symptom. There is no need to treat in this form but need to check out by the regular radiographs.
1 The infected form can be caused by implant installation next to the preexisted infected lesion,
9,
10 implant contamination,
9,
11,
12 bone necrosis related to overheating or other injury
13,
14 and bony microfracture due to premature loading.
9 This form usually has various clinical symptoms such as pain, induration, swelling and fistula formation.
7
IPL can be also subclassified into type I (from implant to tooth) and type II (from tooth to implant) depending on pathologic pathways.
15 Possible causative factors for type I can be too short distance between drilling site and the tooth, overheating, direct damage on the root and disturbing blood supplying to the pulp.
6,
16-
18 Type II lesion can be formed when installed implant contacts with the periapical lesion originated by adjacent non-vital tooth.
In general, treatment strategy of IPL depends on the source of infection. For example, if IPL was caused by adjacent periapical lesion related to nonvital tooth, it should be required to treat adjacent natural tooth as well as implant lesion. Mombelli and Lang
19 suggested that systemic antibiotic therapy could be required if stable implant showed suppuration. The clinical condition could be significantly improved after systemic antibiotic therapy without any additional treatment. They recommended antibiotic agents such as metronidazole which would act against anaerobic bacteria in IPL case. Kao et al.
20 suggested the combined administration of amoxicillin and metronidazole for ten days could be effective.
Some researchers showed several successful reports in regard to systemic antimicrobial therapy combined with surgical approach using bone graft materials with or without barrier membrane in infected form of IPL.
7,
11 However, these combined therapies are simply based on some case reports or clinical experiences, not being established yet specifically. The main purpose of the surgical treatment in IPL is the removal of granulation tissue and bacteria on the surface of the involved implant.
If the infected area is only limited to apical area not compromising the supporting bone length around implant, the implant can be retained with the resection of infected implant apex (Implant apicoectomy). If the lesion is small and inactive, there is no need to resect preventively.
7 The most determining factor could be the complete debridement of the involved lesion. Implant apicoectomy procedure is one of the effective therapies to maintain IPL involved implant providing stable osseointegration without additional complications.
3 According to Reiser and Nevins,
7 however, it is recommended that the implant presenting the increased mobility and periapical radiolucency should be removed not to jeopardize any structures around it.
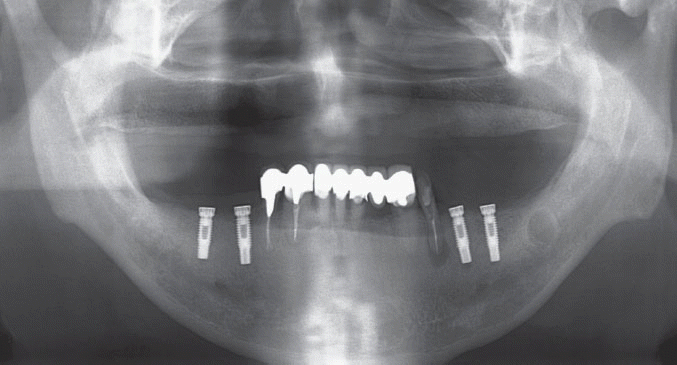
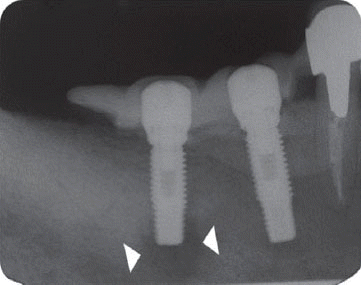
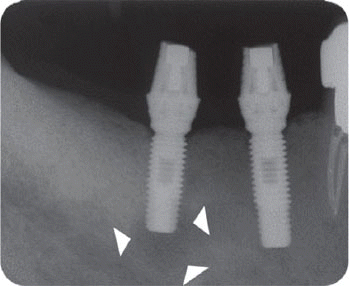
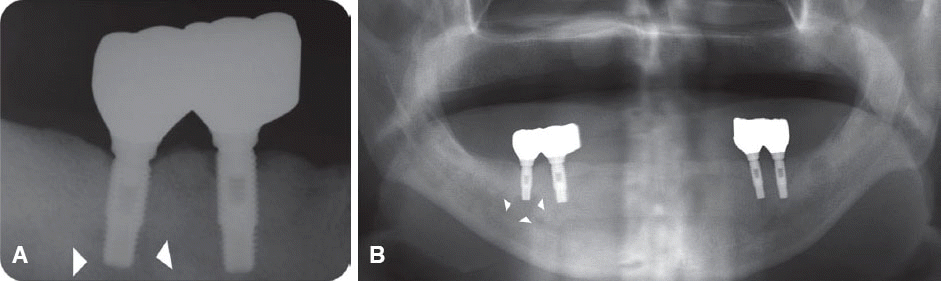
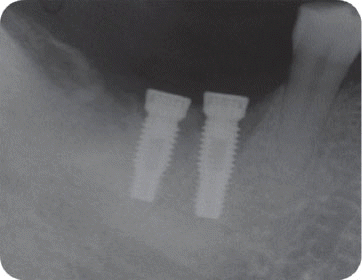
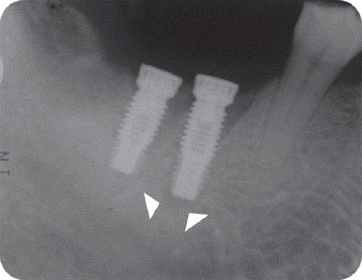
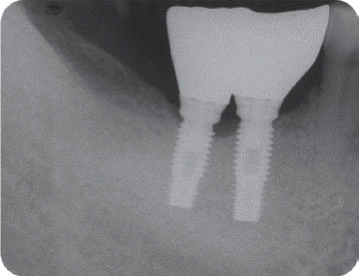
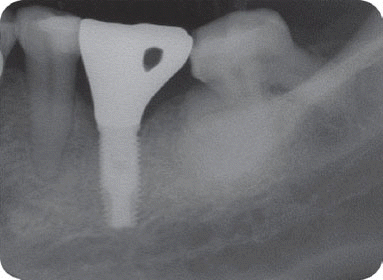
We classified the cases into either the infected or the inactive form depending on the presence of the clinical symptoms in this study. In the first patient case, we determined it as the inactive form of IPL and no treatment was done because there was neither clinical symptom nor functional problem. Although the radiolucency has continued to exist, there was no clinical problem with good stability up to the most recent twelve year-follow up.
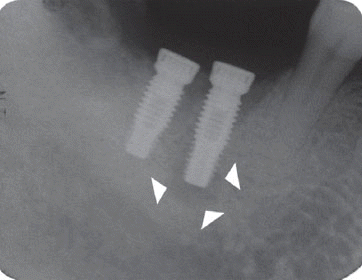
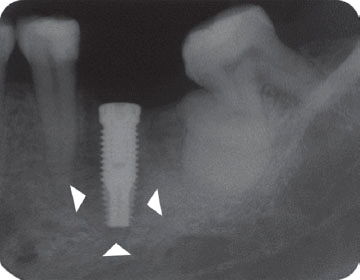
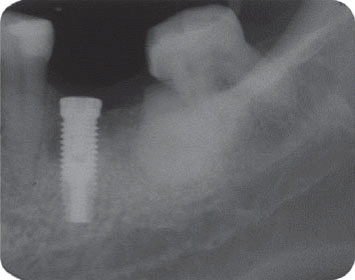
However, the second patient had been under the systemic antibiotic therapy for three weeks until the clinical symptom disappeared because we determined it as the infective form with stable implant. As the pain subsided after systemic antibiotic therapy without any additional treatment, he was planned to be checked up periodically and the five-year follow up radiograph demonstrated the complete disappearance of the radiolucency.
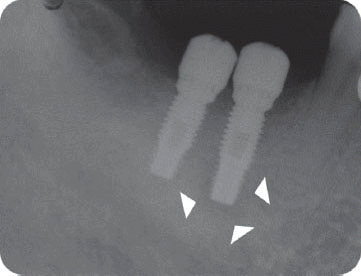
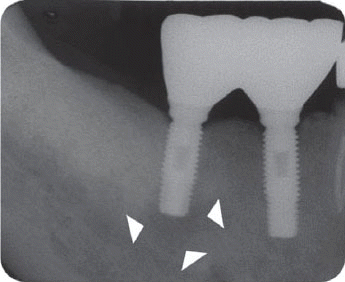
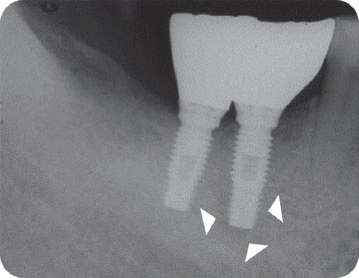
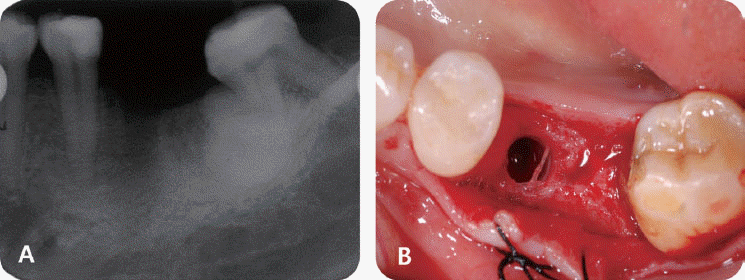
In the third patient case, we also determined it as infected form because it produced pain and clinical symptoms and had the patient under the systemic antibiotic therapy for two weeks. In spite of that, the size of IPL increased on a radiograph and the patient still complained of remaining discomfort. As the next step, we chose the systemic antibiotic therapy combined with surgical approach. However, the implant failed to maintain stability after the surgical therapy and was removed later as suggested by Reiser and Nevins.
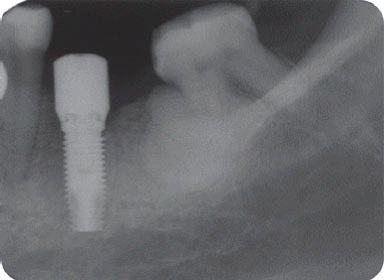
7 Implant was replaced at the same area three months after the explantation, which is showing good stability without any complication by the time of the six-year follow up.
Go to :





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download