INTRODUCTION
Various treatment modalities are used for the first-line treatment of advanced hepatocellular carcinoma (HCC) with portal vein invasion, such as systemic chemotherapy, transarterial chemoembolization (TACE), transarterial radioembolization (TARE), and concurrent chemoradiotherapy (CCRT).
1 These methods are used differently depending on the patient’s characteristics and the treatment experience of each hospital.
2 Although the overall survival rate of these methods is reported variously in each study; they are all methods performed for palliative purposes.
1,
3 If tumor downsizing is sufficiently achieved with initial treatment, active treatment, such as surgery, should be considered for a curative purpose.
4 However, careful judgment is required when deciding on surgery. This is because surgery beyond the Milan criteria may result in tumor recurrence and a poor prognosis, and post-hepatectomy liver failure is likely to occur if the future liver remnant is small.
4–
6
Herein, we report a case of advanced HCC in which tumor size decreased by locoregional therapies was effectively treated with “associating liver partition and portal vein ligation for staged hepatectomy (ALPPS)”. This case report is described according to the CARE guidelines available from
https://www.care-statement.org/. The study was approved by the Institutional Review Board (IRB) of Gangnam Severance Hospital (IRB No. 3-2021-0481), and the requirement for informed consent from the patient was waived.
Go to :

CASE REPORT
A 47-year-old male undergoing follow-up observation at another hospital for hepatitis B virus cirrhosis visited the hospital for HCC on computed tomography (CT).
He had no liver diseases other than cirrhosis caused by hepatitis B infection. The patient had no family history of HCC or other malignancies. No specific symptoms were observed during the visit. CT revealed 8.2 cm HCC in S8 and 1.6, 1.4 cm in S8 and 1.7 cm intrahepatic metastases in S6 (
Fig. 1). On the initial complete blood count panel, the following were observed: white blood cell count, 6.34×10
3/μL; hemoglobin level, 15.5 g/dL; and platelet count, 204×10
3/μL. A liver function test revealed a serum bilirubin level of 0.5 mg/dL, albumin level of 4.2 g/dL, and prothrombin time international normalized ratio of 1.01. Furthermore, the serum alpha-fetoprotein (AFP) level was 4,036 ng/mL, while the protein induced by the absence of vitamin K or antagonist-II (PIVKA-II) was 2,993.6 mAU/mL. Magnetic resonance imaging (MRI) revealed a 9 cm hepatic mass across segments 7 and 8, including enhancement in the arterial phase and washout in the portal and delayed phases. Approximately 1.7 cm of intrahepatic metastases were observed in S6 and S8, and approximately 1.5 cm non-specific lymph node enlargement was observed in the porta hepatis, portal space, and para-aortic area; portal vein invasion of S8 was observed, and infiltration was suspected in the middle hepatic vein (
Fig. 2). Chest CT and whole-body bone scan showed no extrahepatic tumor metastasis.
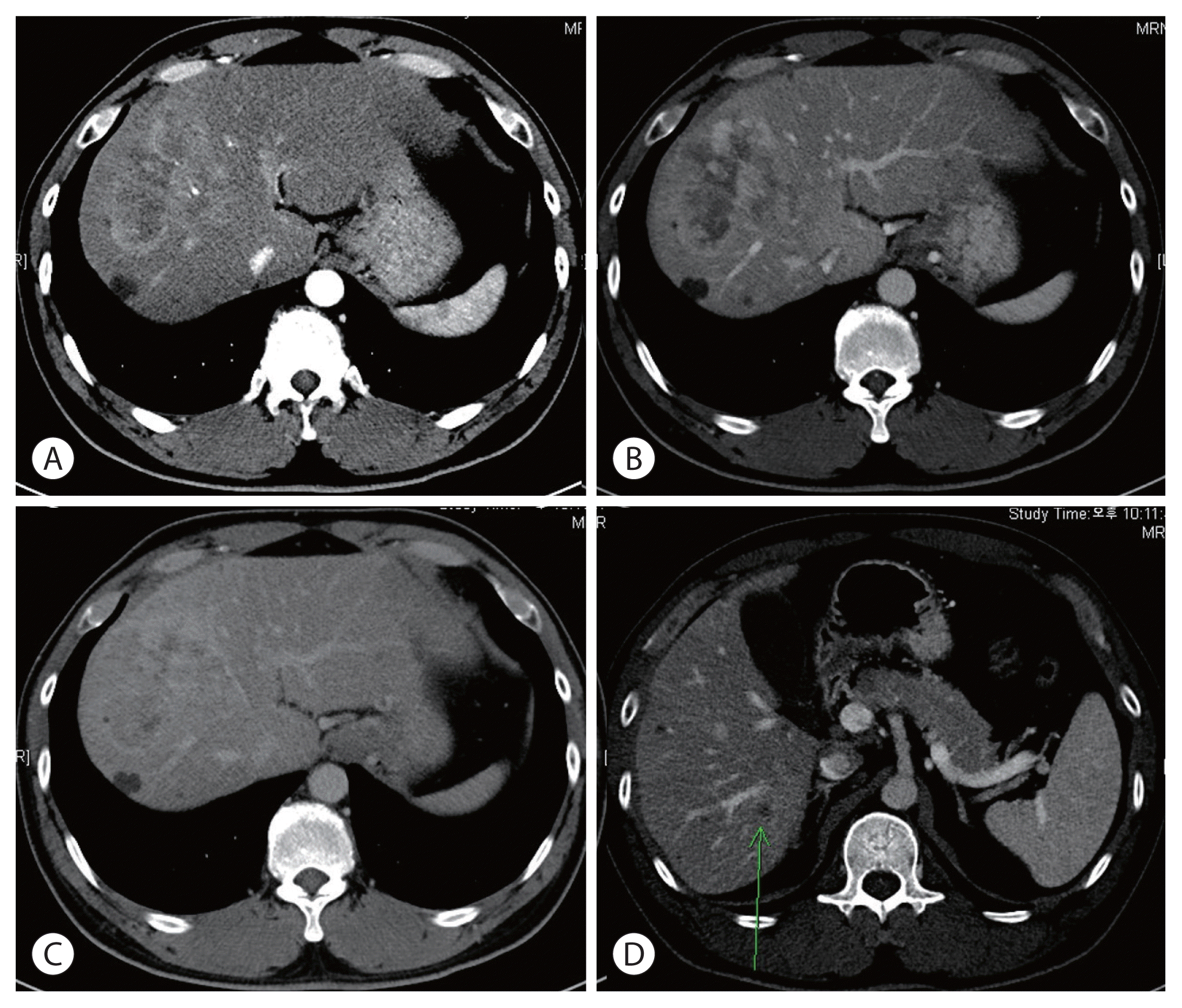 | Figure 1Dynamic liver computed tomography at the time of diagnosis. (A) Arterial phase, (B) portal phase, and (C) delayed phase image. (D) Intrahepatic metastasis in S6 (green arrow) is observed. 
|
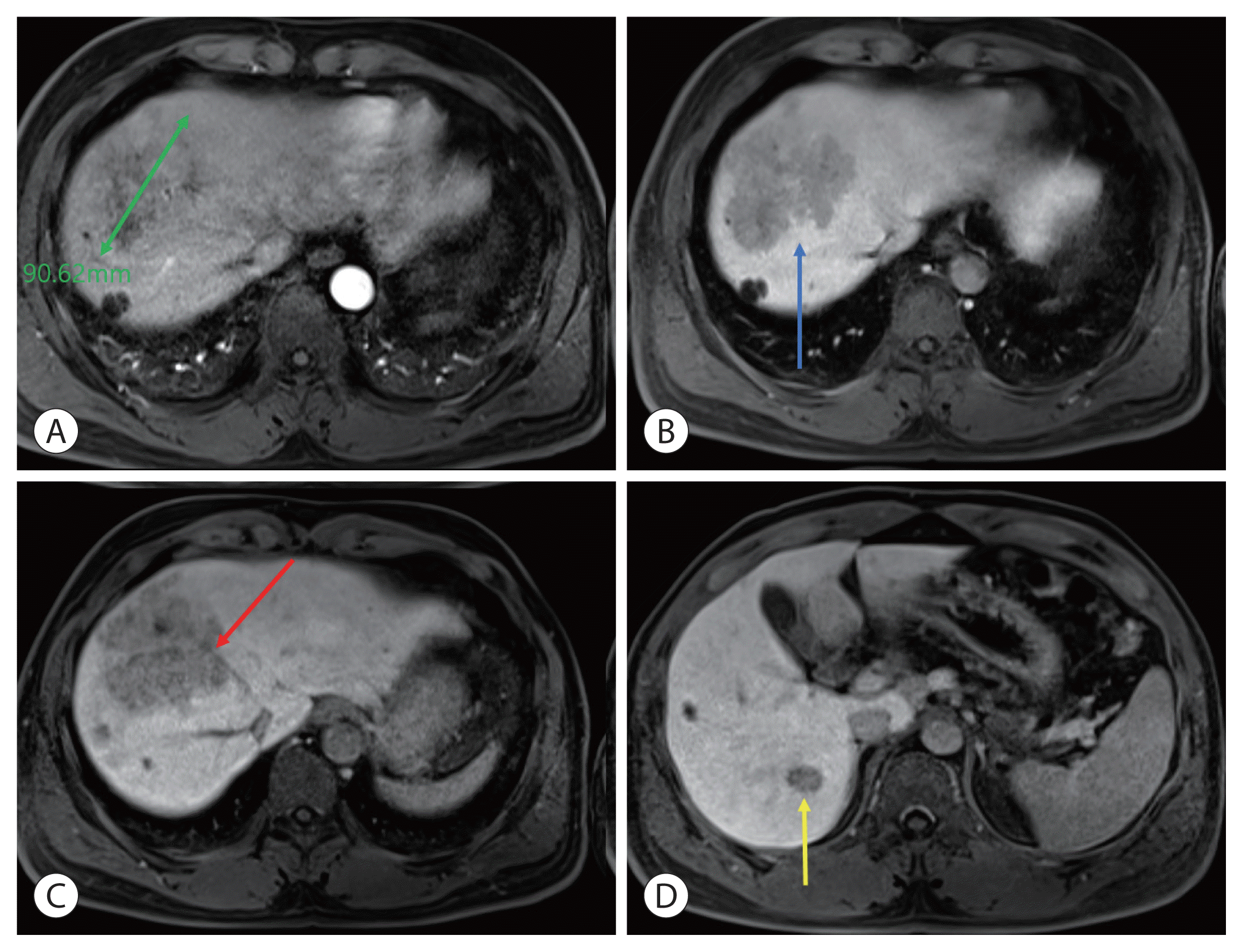 | Figure 2Liver magnetic resonance imaging at the time of diagnosis. (A) Arterial phase showed a 9 cm enhancing mass in the S8 liver, (B) portal phase showed S8 portal vein infiltration (blue arrow), and (C) delayed phase showed a mass adjacent to the middle hepatic vein (red arrow). (D) Intrahepatic metastasis in S6 (yellow arrow) is observed in the delayed phase. 
|
The patient had a Child-Pugh score of 5 and a performance status score of 0. HCC was classified as an advanced stage according to the Barcelona Clinic Liver Cancer system and as stage IVa (T4N0M0) according to the modified Union for International Cancer Control TNM classification. Liver function is relatively well preserved; however, advanced stages accompanied by portal vein invasion, middle hepatic vein infiltration, and multiple intrahepatic metastases have significant restrictions on the selection of treatment options. Therefore, CCRT was performed for locally advanced HCC to reduce the size of the main target lesion and treat the surrounding metastatic lesions. Radiotherapy involved 62.5 Gy in 25 fractions, including the left liver mass. During this period, hepatic arterial 5-fluorouracil was administered during the first and 5th weeks of RT at a dose of 500 mg/day. After 5 weeks of CCRT, CT showed a decrease in the HCC size. Serum tumor marker levels also markedly decreased after CCRT. Serum AFP and PIVKA-II levels dramatically decreased from 4,036 to 440.6 ng/mL and 2,993.6 to 62.9 mAU/mL, respectively. CCRT successfully reduced the tumor size, and hepatic artery injection chemotherapy (HAIC) was maintained four times every month. The anticancer drug was administered once a month through the hepatic artery for 3 days. The 5-fluorouracil was administered at a dose of 500 mg/day for 3 days (days 1, 2, and 3), and cisplatin was added at a dose of 80 mg/day for 1 day (day 2). After four HAIC sessions, MRI was performed. The size of the main lesion slightly decreased from 9.0 to 7.4 cm, the size of independent intrahepatic metastases did not change, and portal vein infiltration was still observed (
Fig. 3). However, AFP decreased dramatically from 4,036 to 8.0 ng/mL before treatment and PIVKA-II from 2,993.6 to 47.4 mAU/mL. After CCRT and sequential HAIC were judged to be effective, hepatectomy was considered as curative treatment. However, because the size of the left liver is relatively small, we decided to proceed with ALPPS, which induces an increase in the size of the left liver after ligation of the right portal vein. In the first stage, ligation of the right portal vein and S4 branch of the portal vein was performed. To prevent blood flow between the right hemiliver with the main lesion and the left hemiliver without the lesion, the hepatic compartment was separated from the falciform ligament to the vena cava. After separation, an antiadhesive material (Sepafilm) was attached to the separated surface to prevent reperfusion. On CT performed 1 month postoperatively, the size of the left hemiliver did not increase significantly, and an increased amount of ascites was discovered. Furthermore, ICG R15 was 23.6%, and there was a risk of liver failure in the second stage of the operation; therefore, regular follow-up was performed. At regular follow-up, there was no evidence of HCC progression according to the mRECIST criteria, and there was no change in the tumor markers AFP and PIVKA-II; therefore, another bridging therapy was not considered. On CT performed 10 months after the first operation, the main lesion at S7 and 8 was approximately 4 cm, and the size was reduced by more than half. There was no intrahepatic metastasis or newly discovered extrahepatic lesions. The previously observed ascites disappeared, and the size of the left hemiliver increased significantly from 16.6 to 22.2 cm (
Fig. 4). Therefore, we decided to proceed with the second-stage operation, right trisectionectomy was performed, and the patient was discharged without complications. In the resected specimen, infiltration of foamy histiocytes was observed into the necrotic tissue and periphery, but there was no evidence of viable cancer. There was no recurrence or metastasis on follow-up CT after second-stage surgery. On follow-up CT after 16 months, no recurrence of intrahepatic HCC or extrahepatic metastasis was observed (
Fig. 5). AFP 1.8 ng/mL and PIVKA-II 27.6 mAU/mL were all within the normal range and a complete response has been maintained to date (
Fig. 6).
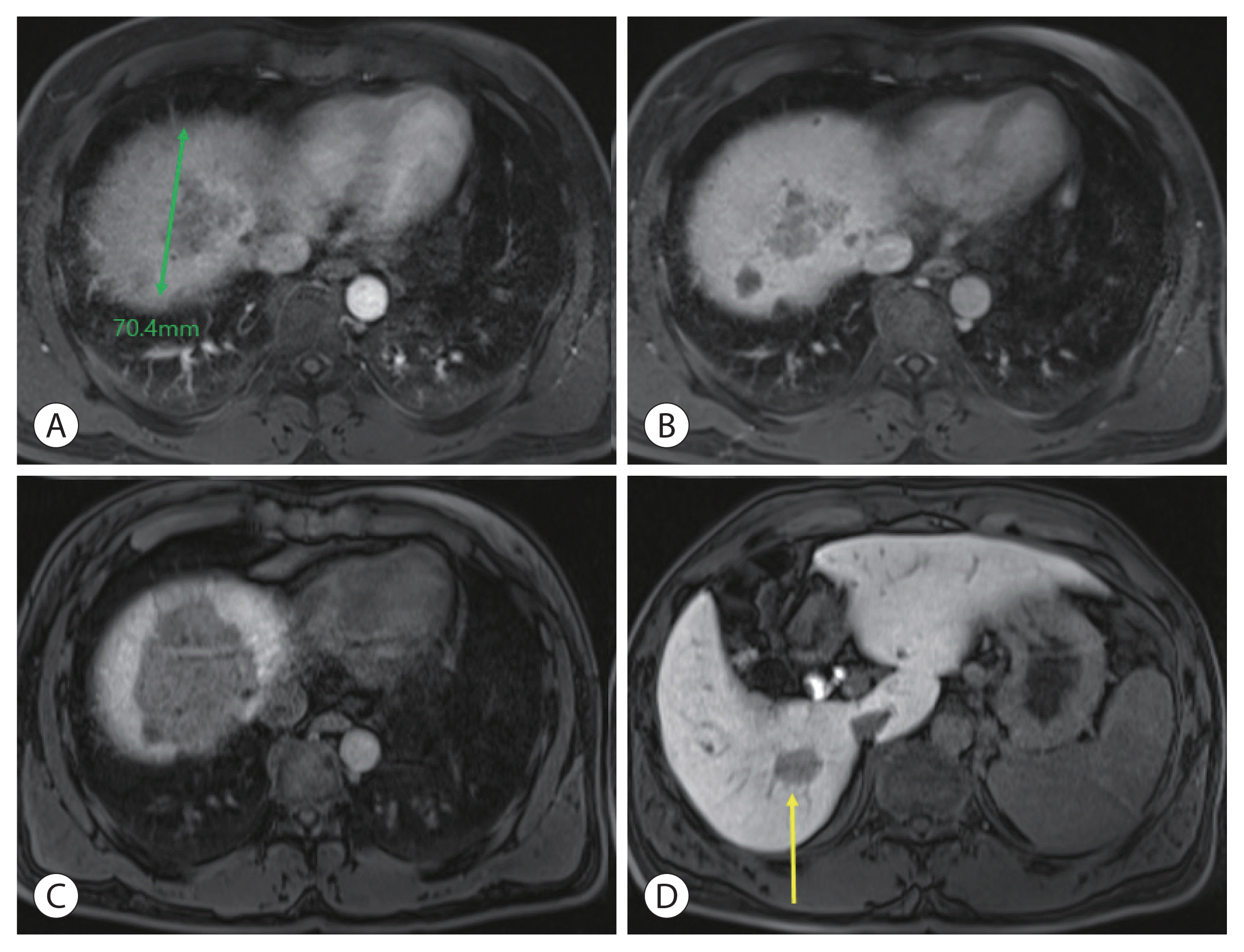 | Figure 3Liver magnetic resonance imaging after concurrent chemoradiotherapy and sequential hepatic artery infusion chemotherapy. The size of the main mass decreased to 7 cm in the (A) arterial phase, (B) portal phase, and (C) delayed phase. (D) The size of the S6 independent lesion (yellow arrow) showed no change in the delayed phase. The main lesion is slightly reduced, but stable disease status overall, according to mRECIST criteria. 
|
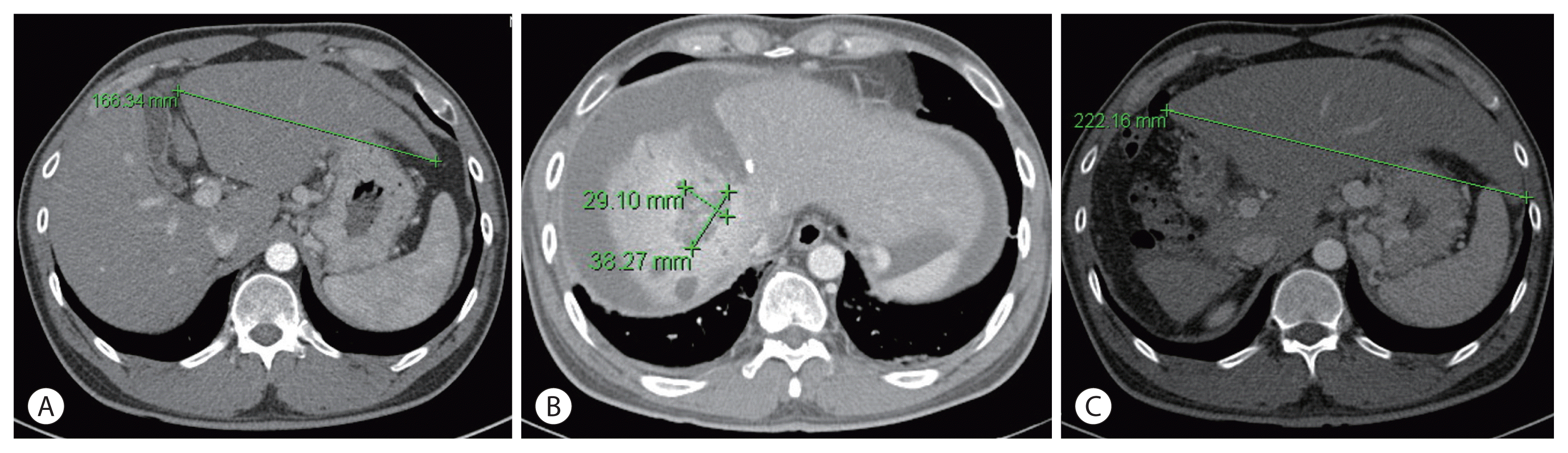 | Figure 4Comparison of computed tomography images before and after portal vein ligation of the right portal vein and S4 branch. (A) The size of the left liver before portal vein ligation. (B) Ascites occurred 1 month after ligation. (C) Increased size of the left liver 10 months after ligation. 
|
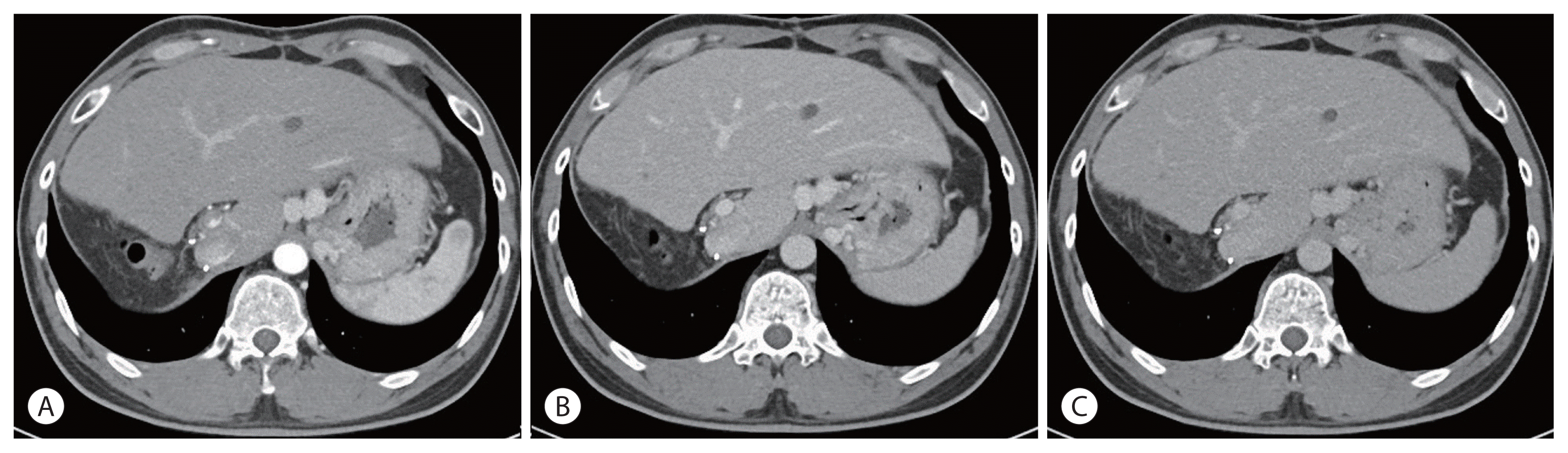 | Figure 5Dynamic liver computed tomography 16 months after associated liver partition and portal vein ligation for staged hepatectomy. Complete remission was maintained as seen in the (A) arterial phase, (B) portal phase, and (C) delayed phase. 
|
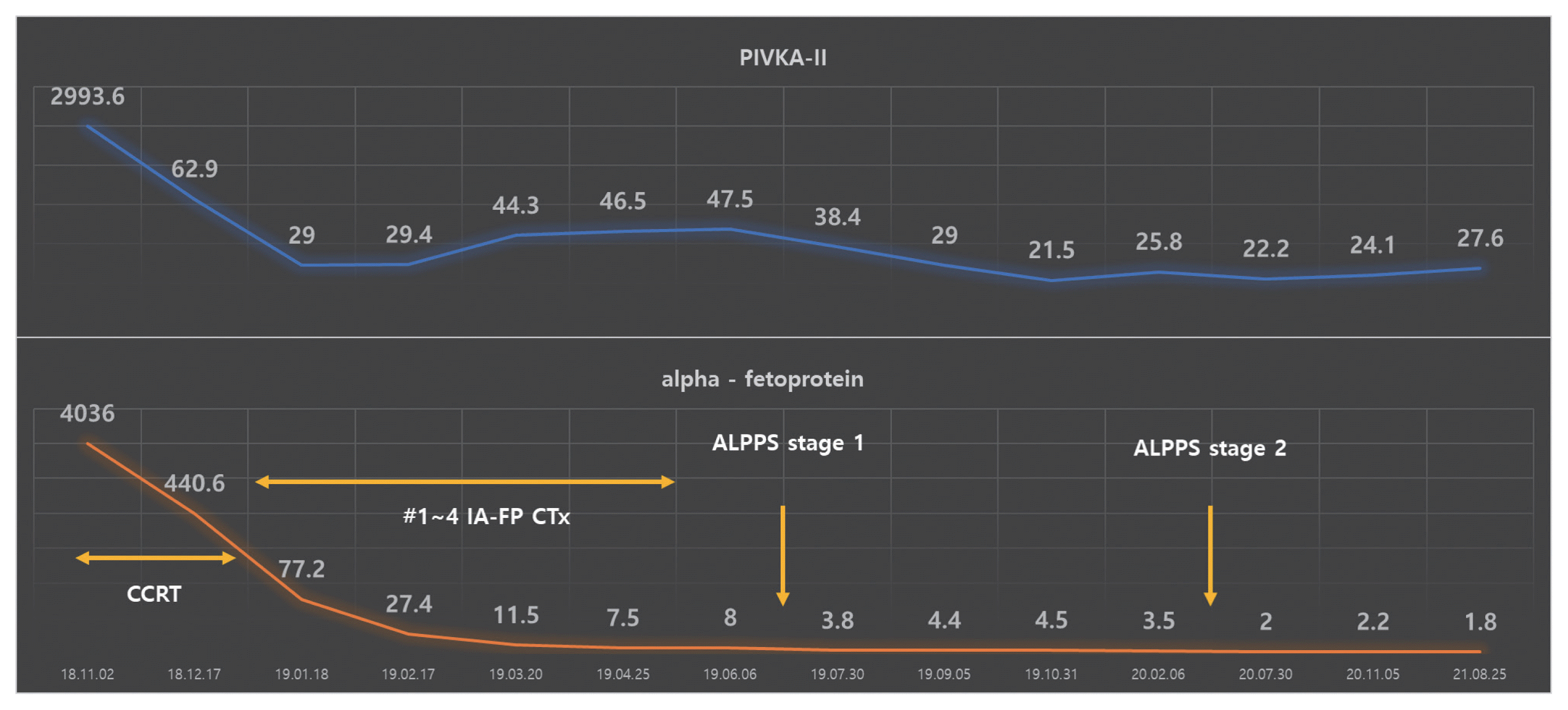 | Figure 6Tumor markers during the total follow-up period from the time of diagnosis. Both AFP and PIVKA-II decreased. AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence or antagonist-II; ALPPS, associating liver partition and portal vein ligation for staged hepatectomy; CCRT, concurrent chemoradiotherapy; IA-FP CTx, intra-arterial 5-fluorouracil and cisplatin chemotherapy. 
|
Go to :

DISCUSSION
According to the 2018 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines, there are various treatment methods for unresectable HCC with BCLC (C, advanced stage), AJCC IIIB, modified UICC IVa, CTP score A, and performance status 0. Systemic chemotherapy using sorafenib, lenvatinib, TACE with external beam radiation therapy, drug-eluting beads-TACE (DEB-TACE), or TARE using Yttrium-90 (
90Y) microspheres can be considered as first-line treatment.
7 Conventional TACE, DEB-TACE, and TARE are therapeutic methods that selectively access blood vessels that supply blood to cancer cells, causing embolism and necrosis of cancer cells.
8 Compared to TACE, DEB-TACE uses higher concentrations of drugs within target tumors and lower systemic concentration. Therefore, the use of DEBs can reduce drug-related adverse events such as post-embolization syndrome.
9 TARE is a method of using radioactive isotope, β-ray emitting Yttrium-90, that not only can induce necrosis by blocking only simple blood supply but also maximize the therapeutic effect given by radiation.
10
As another method, the combination treatment of HAIC and external beam radiation therapy showed a median survival of 13–20 months.
11 Based on this report, there is no difference from the median survival period of systemic chemotherapy, and it has the advantage of considering surgical treatment when downstaging is performed.
12
In this case, although the main lesion was HCC confined to the liver at the time of diagnosis, it occupied most of the right lobes at S7 and 8. In addition, intrahepatic metastases in S6 and invasion of the right portal vein were observed, making resection impossible, and liver transplantation was not considered. Therefore, CCRT over 5 weeks and HAIC four times at 1-month intervals were performed, and the size of the main mass in the right lobe was significantly reduced by 4 cm. Intrahepatic metastases were not well delineated, suggesting improvement, and active surgical treatment was considered for curative purposes. However, when the right liver was resected, the size of the left liver was <30% of the total liver size, and the risk of hepatic failure after surgery was high. Therefore, after ligation of the right portal vein (stage 1 surgery), an increase in the size of the left liver was induced, and curative resection (stage 2 surgery) was performed.
This surgical method is called ALPPS and was first tried in Germany in 2007.
13 This technique was used mainly for hepatic metastasis of colorectal cancer.
14 It can be tried only when one large lesion or several small lesions are confined to one lobe, and the size of the remaining liver is small.
15 The advantage of ALPPS is that it enables rapid hypertrophy of the future liver remnant and reduces the risk of post-hepatectomy liver failure.
16 The disadvantage of this treatment is that the size of the remaining liver does not increase unexpectedly after portal vein ligation, which carries the risk of cancer progression while waiting for it to grow.
16 Another disadvantage involves the risk of liver failure after portal vein ligation, leading to sepsis and multiple organ failure.
17 In this patient, after right portal vein ligation (stage 1 surgery), the pressure of the portal vein temporarily increased, and ascites occurred. After ligation of the right portal vein, the left lobe grew sufficiently for a relatively long period of 10 months. Fortunately, the ascites disappeared during that time, no other metastases were observed, and curative resection (stage 2 surgery) was performed effectively.
To date, the effectiveness of the two-stage ALPPS technique for long-term survival is unknown. Although it is a surgical technique that goes beyond existing guidelines, it can provide new hope to patients who can only expect palliative treatment, including chemotherapy. However, it is unknown which patient characteristics should be selected and applied. In cases of poor initial treatment response or severe liver cirrhosis, aggravation of the disease may occur. The HCC of the patient responded well to the first-line treatment, CCRT, and HAIC, and the size of the lesion was significantly reduced.
After portal vein ligation (stage 1 surgery), the size of the non-tumor liver increased appropriately, although the rate was slow. This is a case of complete remission at 16 months after receiving curative resection (stage 2 surgery) without recurrence or metastasis, although the size of the nontumor liver grew slowly over 10 months due to cirrhosis. Therefore, curative resection can be considered after HCC downstaging, and ALPPS can be considered for curative resection when the future liver remnant is small.
Go to :











 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download