Abstract
Treatment options for advanced hepatocellular carcinoma (HCC) have been rapidly evolving. Herein, we describe a patient with advanced HCC and portal vein tumor thrombosis (PVTT) who responded decisively to a multidisciplinary approach. The patient had an ill-defined infiltrative HCC (diffuse subtype), with several intrahepatic metastasis and tumor invasion of left portal vein. Concurrent use of transarterial radioembolization (TARE) and systemic therapeutics (atezolizumab + bevacizumab) ultimately proved successful. There was marked reduction in tumor volume after TARE and an additional three cycles of atezolizumab plus bevacizumab. This concurrent treatment was well tolerated, without adverse events during immunotherapy. The impressive results achieved suggest that concurrent TARE and combination atezolizumab/bevacizumab is a promising treatment approach for advanced HCC with PVTT.
Primary liver cancer is the sixth most common malignancy worldwide and the third leading cause of cancer-related mortality. Hepatocellular carcinoma (HCC) accounts for the overwhelming majority (90%) of cases.1 Unfortunately, a substantial number of patients are diagnosed at advanced stage, so the number of actual candidates for potentially curative treatments is limited.2
Recently, a combination regimen of atezolizumab plus bevacizumab received approval as first-line treatment of advanced HCC, based on the IMbrave 150 phase III randomized clinical trial.3 In this study, atezolizumab/bevacizumab duotherapy conferred significantly better overall survival (OS) and progression-free survival (PFS) than did sorafenib. Nevertheless, the objective response rate was only 27.3%, indicating a continued need for novel treatment strategies.
Transarterial radioembolization (TARE) is a novel locoregional therapy using injectable yttrium-90 (90Y)-loaded radioembolic microspheres. Guidelines of both the European Association for the Study and the Liver and the American Association for the Study of Liver Diseases presently stipulate locoregional therapies for Barcelona Clinic Liver Cancer (BCLC) stage A or B,4,5 primarily reserving systemic therapy as treatment of choice for advanced cancers (BCLC stage C). Recent studies have nevertheless shown good response rates and safety with use of TARE in late-stage disease, making it a viable treatment option for advanced HCC.6,7
To maximize advantages of both therapeutic modalities, a combination thereof has been tested as a novel approach to advanced HCC. Herein, we report a remarkable treatment response to concurrent TARE and atezolizumab/bevacizumab administration in a patient with advanced HCC and portal vein tumor thrombosis (PVTT).
This case report is described according to the CARE guidelines available from https://www.care-statement.org/. The Institutional Review Board of Seoul National University Hospital approved this study (IRB no H-2112-038-1279) and waived the requirement for informed consent.
A 43-year-old man with fever sought care at a tertiary medical center. There was a history of alcohol abuse (1 bottle of soju, twice weekly) and smoking (30 pack-years), with claims of abstinence from both for the past 3 months. Abdominal-pelvic computed tomography (CT) showed an infiltrative hepatic mass, initially interpreted as abscess, so he received empiric antibiotic treatment. This brought no improvement, prompting liver biopsy and a revised diagnosis of HCC. Because the tumor was advanced, with PVTT, a combination regimen of atezolizumab/bevacizumab was begun. Hepatitis B virus DNA load was 236,000 IU/mL when diagnosed with HCC; therefore, he started tenofovir disoproxil fumarate treatment for hepatitis B-related liver cirrhosis and HCC along with immunotherapy. Once the first cycle was completed, he was referred to our hospital for a second opinion.
Upon arrival, his medical and family histories were otherwise noncontributory, and there were no related symptoms or notable findings by physical examination. Results of baseline laboratory screening were as follows: white blood cells, 5,160/μL; hemoglobin, 13.3 g/dL; platelet count, 417,000/μL; total bilirubin, 0.6 mg/dL; albumin, 4.1 g/dL; prothrombin time/international normalized ratio, 1.14; aspartate aminotransferase, 50 IU/L; and alanine aminotransferase, 50 IU/L. Liver function was preserved (Child-Pugh class A5). He tested positive for hepatitis B surface antigen, harboring a viral DNA load of 30,300 IU/mL. Baseline serum levels of α-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist-II (PIVKA-II) before atezolizumab/bevacizumab treatment were 654.0 ng/mL and 1,470 mAU/mL, respectively. When he was referred to our hospital, after the first cycle of immunotherapy, serum levels of AFP and PIV-KA-II were 588.0 ng/mL and 7,497 mAU/mL, respectively.
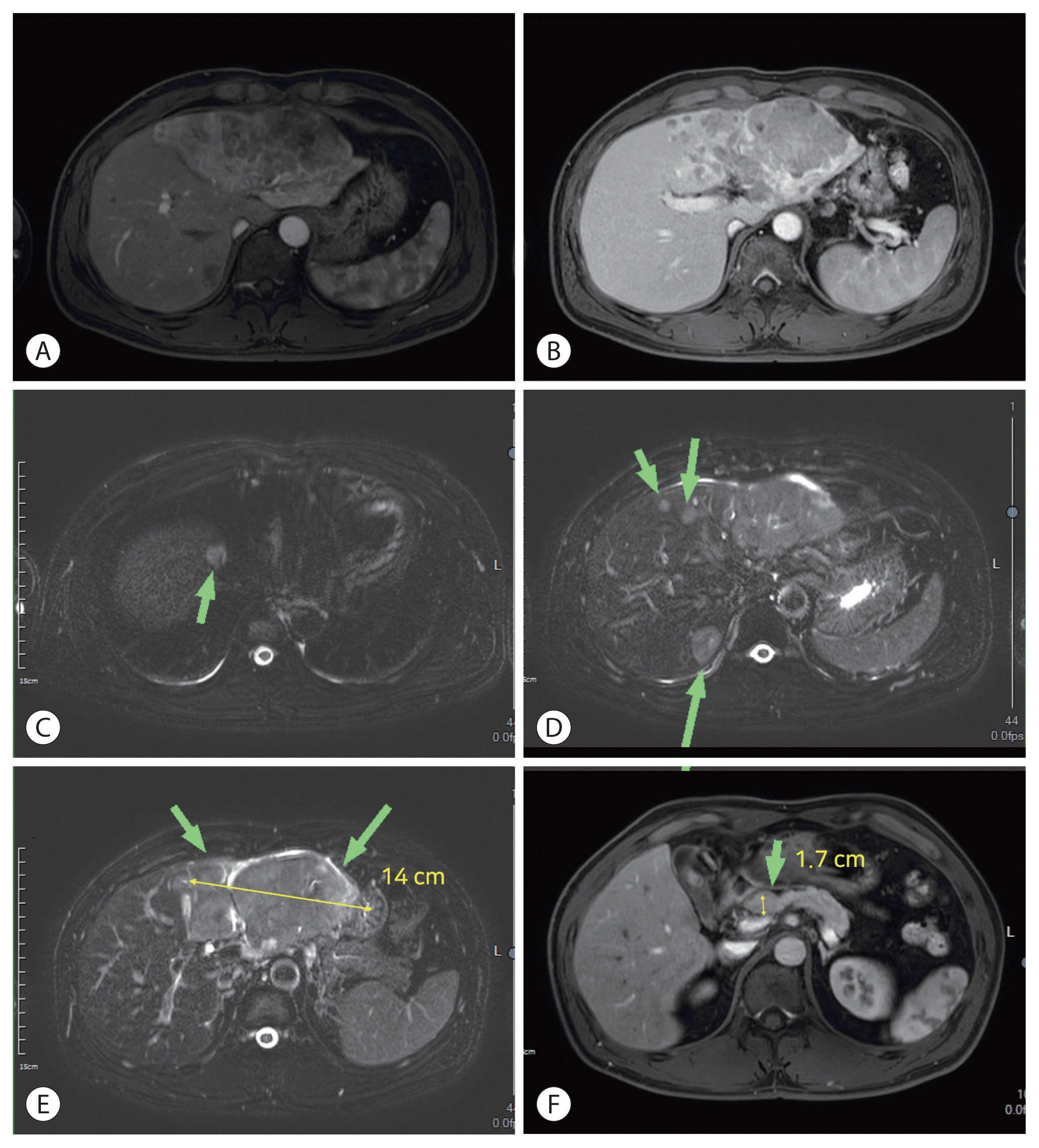
An initially performed contrast-enhanced (Primovist; Bayer AG, Leverkusen, Germany) magnetic resonance imaging study before the first cycle of immunotherapy revealed infiltrative HCC (~14 cm) of the left lobe, with several intrahepatic metastases to right lobe. Tumor thrombosis of left portal vein was also apparent, and a prominent lymph node was noted at common hepatic artery (metastasis not excluded). No other extrahepatic metastases were evident (Fig. 1).
This particular patient was diagnosed with advanced HCC by pathologic and imaging staging (modified Union for International Cancer Control stage IV, T4N1M0, and BCLC stage C). Treatment with atezolizumab (1,200 mg) and bevacizumab (15 mg/kg) was started at another hospital, completing only one cycle before transfer to our facility.
Our plan was to achieve local control of the infiltrative primary tumor (within left lobe) using 90Y TARE. We first ascertained the liver-to-lung shunt fraction, which was 2.38% (i.e., <20%). TARE was undertaken using glass microspheres (TheraSphere®; Boston Scientific, Marlborough, MA, USA) at a mean target tissue dose of 360 Gy and virtual tumor dose of 485 Gy. One day later, the patient developed high fever (up to 38.3°C), without signs of infection. This subsided uneventfully. A cycle of atezolizumab/bevacizumab was administered 5 days after the TARE procedure.
Two weeks after TARE, his liver function was stable. As stated in Common Terminology Criteria for Adverse Events (version 5), no other adverse events were encountered.8 We chose to continue the atezolizumab/bevacizumab regimen, given a potential for synergism between locoregional and systemic therapies.
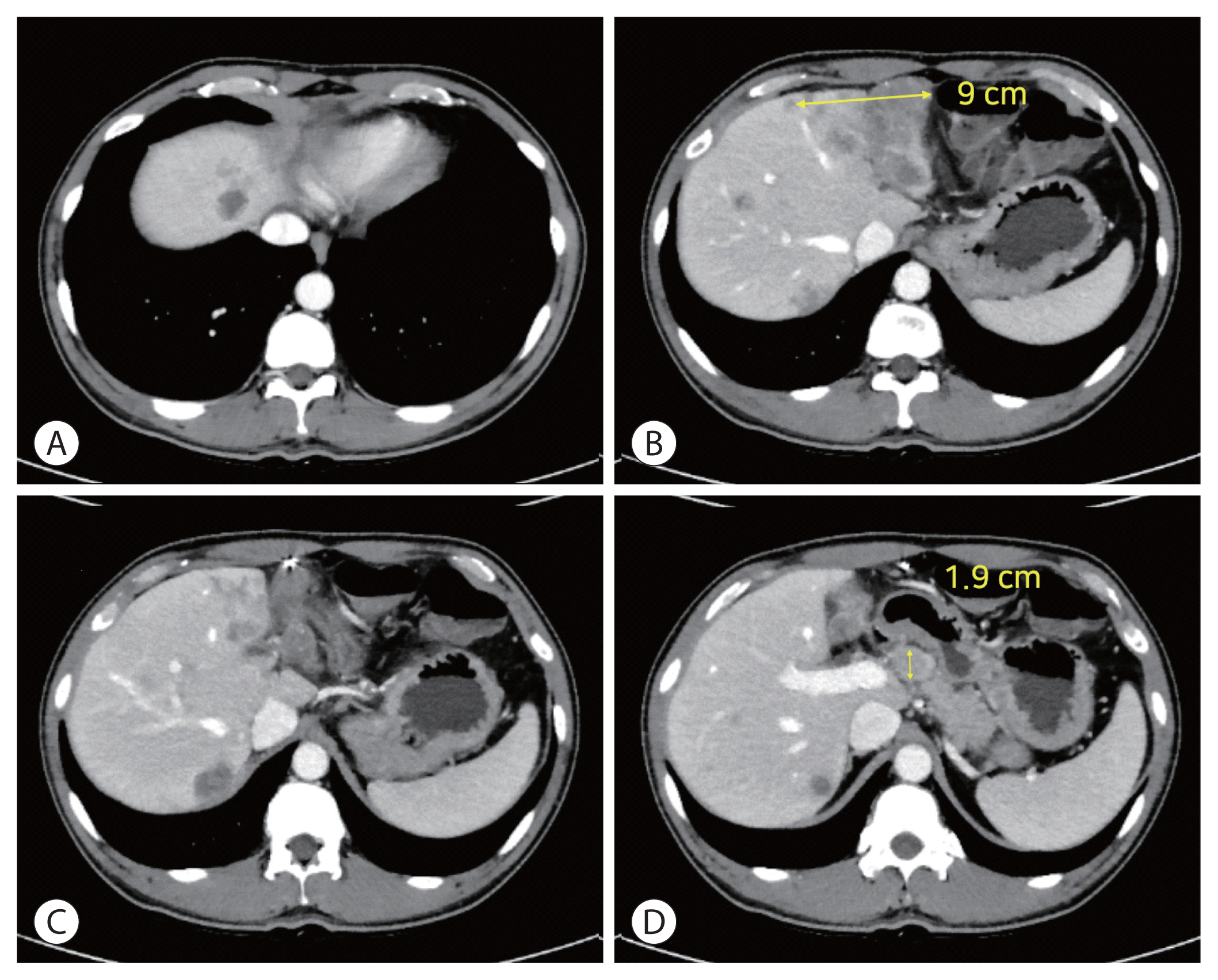
After a third cycle of immunotherapy, treatment response was formally evaluated. We found a significant reduction in size of the invasive left-lobe tumor, its maximum diameter shrinking from 14 to 9 cm on liver CT. The enhanced portion specifically showed remarkable regression (Fig. 2), representing a partial response by modified Response Evaluation Criteria in Solid Tumor (mRECIST) standards.9 Tumor markers also reflected the positive therapeutic effects; his serum AFP and serum PIVKA-II levels falling from 588.0 to 326.4 ng/mL and from 7,497 to 886 mAU/mL, respectively. The patient tolerated immunotherapy therapy well, experiencing no adverse events or change in liver function.
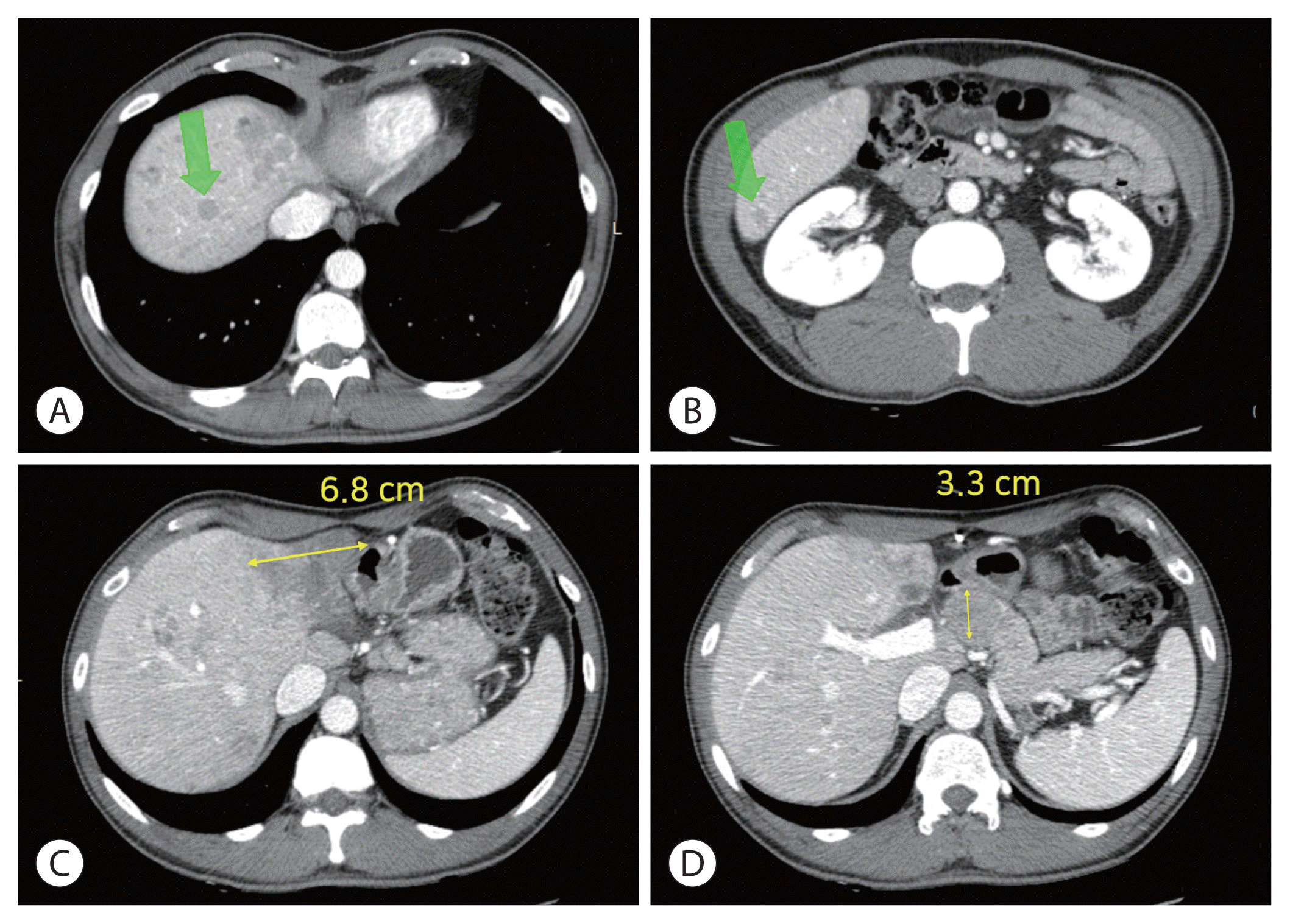
However, after the sixth cycle of immunotherapy, the prominent lymph node at common hepatic artery had enlarged, increasing from 1.9 to 3.3 cm; and new intrahepatic lesions were suspected, signaling progressive disease by mRECIST. Despite further shrinkage of the primary mass (to 6.8 cm) (Fig. 3), tumor markers had risen substantially (AFP, 1,335.2 ng/mL; PIVKA-II, 12,573 mAU/mL). The patient presently awaits participation in a clinical trial of second-line systemic therapy.
Herein, we describe a patient with advanced HCC and PVTT who initially responded well to concurrent locoregional (TARE) and systemic (atezolizumab/bevacizumab) therapies. Although his disease eventually progressed, there was dramatic regression of an infiltrative HCC for a significant time period. The other lesions remained relatively stable, with the exception of a lymph node.
Results of the IMbrave 150 study, a phase III randomized trial, have already demonstrated the superiority of atezolizumab/bevacizumab vs sorafenib in this setting, yielding better OS (stratified hazard ratio [HR], 0.58; 95% confidence interval [CI], 0.42–0.79) and PFS (stratified HR, 0.59; 95% CI, 0.47–0.76).3 Consequently, clinical guidelines of the National Comprehensive Cancer Network now recommend atezolizumab/bevacizumab duotherapy as a first-line treatment for advanced HCC.10
TARE is also considered a valid treatment option in this setting. Thrombosis of the portal vein interdicts its compensatory role during transarterial chemoembolization (TACE) and is thus a relative contraindication for such procedures. Unlike TACE, however, TARE has minimal impact on hepatic arterial occlusion. Moreover, TARE tended to have fewer complications, especially less abdominal pain, and a shorter hospital stay than TACE.11 Since this patient had a large infiltrative HCC in the left lobe, TARE was selected to reduce the complication rather than TACE. In a number clinical trials, TARE and sorafenib have performed comparably as treatment of HCC.12,13 The SARAH phase III randomized trial recruited patients with locally advanced or intermediate-stage HCC. TARE (8.0 months) and sorafenib (9.9 months) achieved similar median OS times (HR, 1.15; 95% CI, 0.94–1.41), although treatment response, quality of life, and safety profiles fared better in the TARE group.12 In another randomized phase III study of Asia-Pacific patients, the SIRveNIB trial, TARE proved comparable to sorafenib in terms of OS but showed better tolerability and a higher tumor response rate.13
There have also been some attempts to combine TARE with systemic treatment. The SORAMIC study tested TARE plus sorafenib vs. sorafenib alone. Whereas combination (vs single-agent) treatment failed to significantly improve OS, subgroup analysis underscored a potential for survival benefits in younger patients (≤65 years) and in the absence of cirrhosis or alcohol-induced disease.14
On a theoretic basis, use of dual-agent atezolizumab/bevacizumab (rather than sorafenib) is a preferable addition to TARE. Atezolizumab is an immune checkpoint inhibitor targeting programmed death ligand-1 (PD-L1).3 TARE serves to heighten tumor antigen release and promote an inflammatory tumor microenvironment (TME), boosting the response to atezolizumab.15,16 Expression levels of PD-L1 and other tumor-associated antigens are actually enhanced by radiation. 17 Bevacizumab is a vascular endothelial growth factor (VEGF) inhibitor with potential for anti-PD-L1 synergism. VEGF inhibition helps promote normalization of tumor vessels (mediated by CD4+ cells), thus aiding immune cell recruitment within the TME and augmenting PD-L1 inhibitor efficacy.18 VEGF inhibition also reduces recruitment of immunosuppressive cells (i.e., regulatory T cells or myeloid-derived suppressor cells) that limit the effects of PD-L1 blockade.19 A phase II clinical trial addressing this combined therapeutic rationale is ongoing (NCT04541173).20
In conclusion, our experience with the above patient suggests that concurrent TARE and atezolizumab/bevacizumab duotherapy is an effective and safe treatment regimen for advanced HCC with PVTT. Additional studies are warranted to validate this premise.
Notes
Ethics Statement
The Institutional Review Board of Seoul National University Hospital approved this study (IRB No. H-2112-038-1279) and waived the requirement for informed consent.
Funding Statement
None of the funding sources had any role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–249.

3. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020; 382:1894–1905.

4. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018; 68:723–750.

5. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018; 69:182–236.
6. Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010; 138:52–64.

7. Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011; 54:868–878.

8. Common Terminology Criteria for Adverse Events (CTCAE) version 5. Washington, DC: US Department of Health and Human Services, National Institutes of Health, National Cancer Institute;2017.
9. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010; 30:52–60.

10. Benson AB, D’Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021; 19:541–565.
11. Lau WY, Sangro B, Chen PJ, Cheng SQ, Chow P, Lee RC, et al. Treatment for hepatocellular carcinoma with portal vein tumor thrombosis: the emerging role for radioembolization using yttrium-90. Oncology. 2013; 84:311–318.

12. Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux GP, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017; 18:1624–1636.
13. Chow PKH, Gandhi M, Tan SB, Khin MW, Khasbazar A, Ong J, et al. SIRveNIB: selective internal radiation therapy versus sorafenib in asia-pacific patients with hepatocellular carcinoma. J Clin Oncol. 2018; 36:1913–1921.
14. Ricke J, Klümpen HJ, Amthauer H, Bargellini I, Bartenstein P, de Toni EN, et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol. 2019; 71:1164–1174.

15. Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015; 520:373–377.

16. den Brok MH, Sutmuller RP, Nierkens S, Bennink EJ, Frielink C, Toonen LW, et al. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer. 2006; 95:896–905.

17. Deng L, Liang H, Burnette B, Weicheslbaum RR, Fu YX. Radiation and anti-PD-L1 antibody combinatorial therapy induces T cell-mediated depletion of myeloid-derived suppressor cells and tumor regression. Oncoimmunology. 2014; 3:e28499.

18. Shigeta K, Datta M, Hato T, Kitahara S, Chen IX, Matsui A, et al. Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology. 2020; 71:1247–1261.

Figure 1
Initial dynamic magnetic resonance imaging study of liver: (A) large infiltrative hepatocellular carcinoma, enhanced on arterial phase; (B) washout on portal phase; (C–E) multiple intrahepatic metastases (arrows); and (F) prominent lymph node at common hepatic artery (arrow).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download