CASE REPORT
The first case was a 66-year-old man who visited our clinic complaining of abdominal distension and weight loss of 7 kg over the previous 3 months. He had hypertension and chronic hepatitis B infection and was being treated with 300 mg tenofovir disoproxil fumarate once daily for several years. On physical examination, he presented with tenderness in the right upper quadrant.
Complete blood count showed hemoglobin level of 7.0 g/dL, leukocyte count of 6,500/μL, and platelet count of 153,000/μL. Laboratory examinations showed serum aspartate aminotransferase (AST) 44 IU/L, alanine aminotransferase (ALT) 32 IU/L, alkaline phosphatase (ALP) 216 IU/L, gamma-glutamyltransferase (GGT) 49 U/L, total bilirubin level 0.62 mg/dL, total protein 7.60 g/dL, and albumin 2.81 g/dL. The international normalized ratio (INR) of prothrombin time was 1.45. Hepatitis B surface antigen and envelope antibody were positive, and the hepatitis B virus DNA level was 477 IU/mL.
Liver dynamic computed tomography (CT) showed that approximately 12.8 cm of the heterogeneously enhanced mass occupied the left lobe in the arterial phase, with washout in the delayed phase. Additionally, a left portal vein thrombus extending to the main portal vein was observed in the portal phase (
Fig. 1). Initial alpha-fetoprotein (AFP) was 538.25 ng/mL (normal range, ≤9 mg/dL) and prothrombin-induced by vitamin K absence or antagonist-II (PIVKA-II) was 146.54 mAU/mL (normal range, ≤40 mAU/mL).
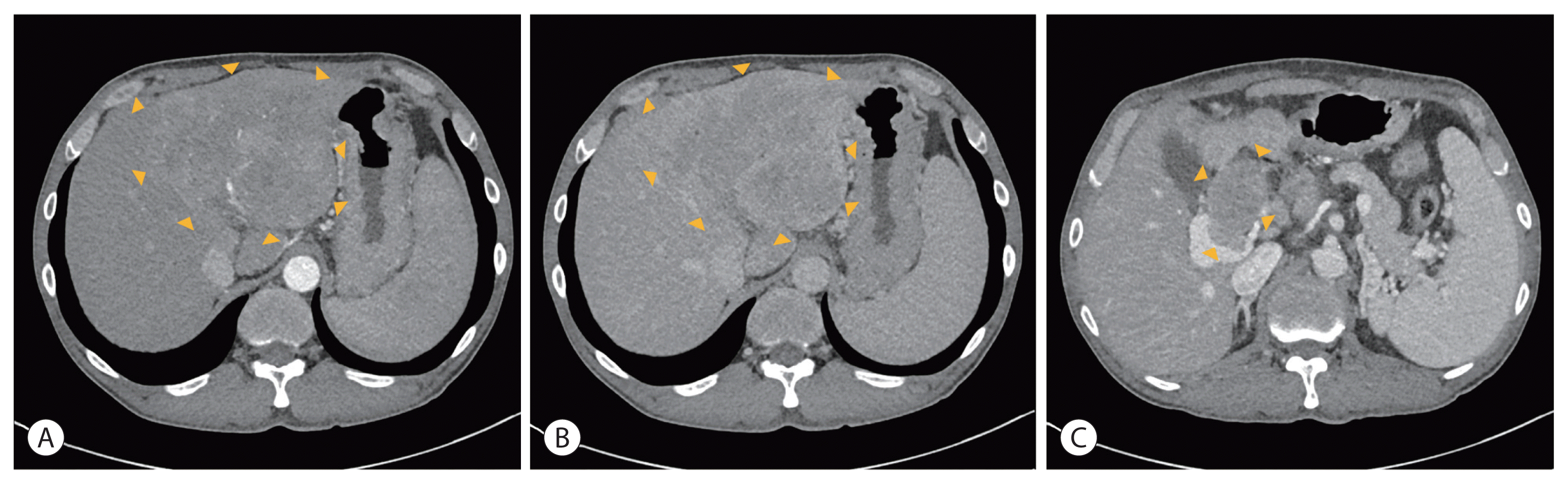 | Figure 1Initial liver dynamic computed tomography of the first patient showed approximately 12.8 cm of the heterogeneously enhanced mass in the arterial phase (A) with washout in the delayed phase (B). The main and left portal vein tumor thrombosis was observed in the portal phase (C). The boundaries of the tumor and PVTT are indicated with arrowheads (A–C). 
|
Based on clinical and imaging findings, the hepatic mass was diagnosed as HCC. Chest CT did not show any evidence of lung metastasis. Child-Pugh score of 6, class A, and Eastern Cooperative Oncology Group (ECOG) score of 0. The patient was classified according to the modified Union for International Cancer Control (mUICC) as stage IVa (T4N0M0) and as advanced stage (stage C) according to the Barcelona Clinic Liver Cancer (BCLC) staging system. We performed esophagogastroduodenoscopy before starting HCC treatment and found esophageal varices of form 0 at the lower esophagus and no gastric varix.
Systemic treatment was recommended as the first-line treatment of mUICC IVa and BCLC C according to the Korea Practice Guidelines for Patients with HCC from the Korean Liver Cancer Association-National Cancer Center 2018 and BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update.
14,
15 Therefore, we chose a treatment regimen including AteBeva, which is a first-line systemic treatment. Simultaneously, we performed HAIC based on 5-fluorouracil (5-FU) and cisplatin, because previous reports have shown that HAIC improved the survival rate in patients with intrahepatic tumors, even in those with extrahepatic metastases or PVTT.
12,
13 In addition, another report has shown that the outcome of advanced stage HCC was better after treatment with HAIC with anti-programmed cell death protein-1 immunotherapy than HAIC alone in terms of overall survival, PFS, disease control rate, and intrahepatic response.
11 In this case, atezolizumab (Ticentriq
®, 1,200 mg per dose; Roche International LLC, Basel, Switzerland) and bevacizumab (Avastin
®, 15 mg/kg per dose; Genentech, South San Francisco, CA, USA) were administered 1 day every 3 weeks. For HAIC, 500 mg/m
2 5-FU for 3 days and 60 mg/m
2 cisplatin for 1 day were administered. HAIC was performed at 3-week intervals on the day after AteBeva administration. Response was evaluated after every three cycles of AteBeva with HAIC treatment.
After the 4th cycle of AteBeva and HAIC, follow-up liver dynamic CT showed decreased size of the intrahepatic mass and left PVTT but an extension of PVTT to the right portal vein, and increased AFP and PIVKA-II levels. Therefore, radiation therapy (RT) was provided to the remaining hepatic mass and PVTT. A total dose of 45 Gy was administered 10 times at 450 cGy each, continuing with AteBeva and HAIC at 3-week intervals.
After the 8th treatment cycle with AteBeva and HAIC with RT, follow-up gadoxetic acid-enhanced liver magnetic resonance imaging (MRI) showed little interval change in the size of the intrahepatic mass and the size and extent of PVTT with no definite enhancing viable area compared with the previous liver image (
Fig. 2). The levels of tumor markers decreased (
Fig. 3). Treatment response was determined as PR based on the modified Response Evaluation Criteria in Solid Tumors, version 1.1 (mRECIST 1.1) for HCC.
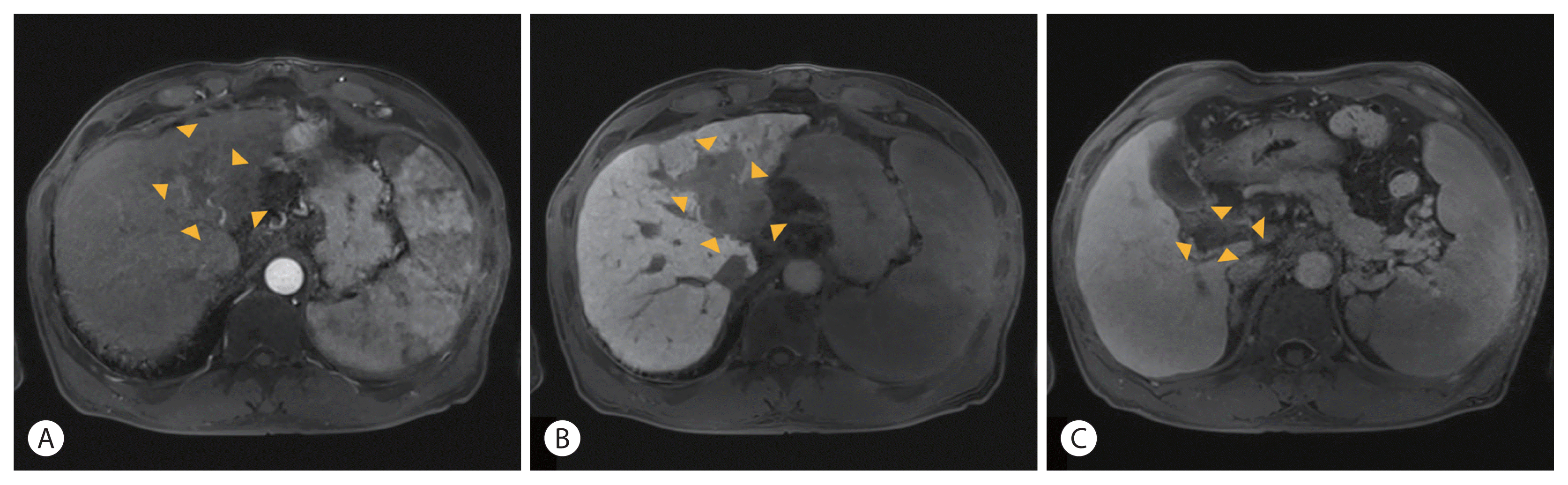 | Figure 2Follow-up gadoxetic acid-enhanced liver magnetic resonance imaging after 8 cycles of atezolizumab plus bevacizumab, hepatic arterial infusion chemotherapy and radiation therapy showed decreased size of the hepatocellular carcinoma without viable area in the arterial and delayed phase (A, B). No extension of portal vein tumor thrombosis was observed in the portal phase (C). The boundaries of the tumor and PVTT are indicated with arrowheads (A–C). 
|
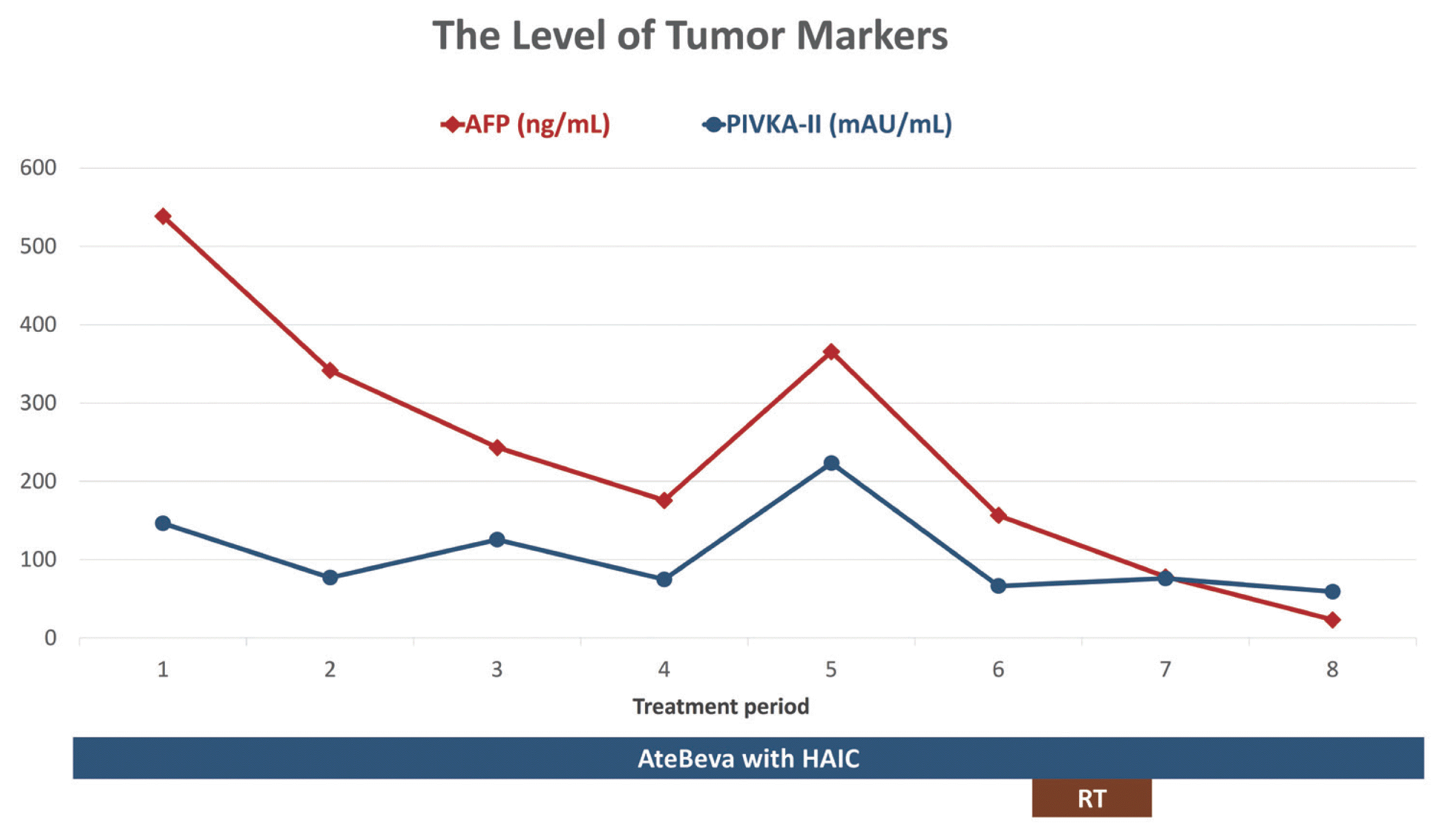 | Figure 3Changes in tumor marker levels in the first patient. AFP, alpha-fetoprotein; PIVKA-II, prothrombin-induced by vitamin K absence or antagonist-II; AteBeva, atezolizumab plus bevacizumab; HAIC, hepatic arterial infusion chemotherapy; RT, radiation therapy. 
|
The second case involved a 54-year-old man who visited our clinic for further evaluation of a hepatic mass and impaired liver function test results after a regular check-up. He had been taking medication for hypertension and dyslipidemia for several years. He was a 45 pack-year current smoker who consumed approximately 600 g of alcohol every week. At the time of presentation, the patient did not experience any abdominal discomfort.
Complete blood count showed hemoglobin level of 9.2 g/dL, leukocyte count of 9,800/μL, and platelet count of 252,000/μL. Laboratory examinations showed serum AST 88 IU/L, ALT 50 IU/L, ALP 78 IU/L, GGT 371 U/L, total bilirubin level 1.01 mg/dL, total protein 7.60 g/dL, albumin 3.62 g/dL, and INR of prothrombin time was 1.31. Hepatitis B surface antigen, core antibody, and hepatitis C viral antibody tests were negative.
Liver dynamic CT showed that approximately 23.5×13.7 cm of the heterogeneously enhanced mass occupied the right lobe in the arterial phase with washout in the delayed phase and no evidence of PVTT (
Fig. 4).
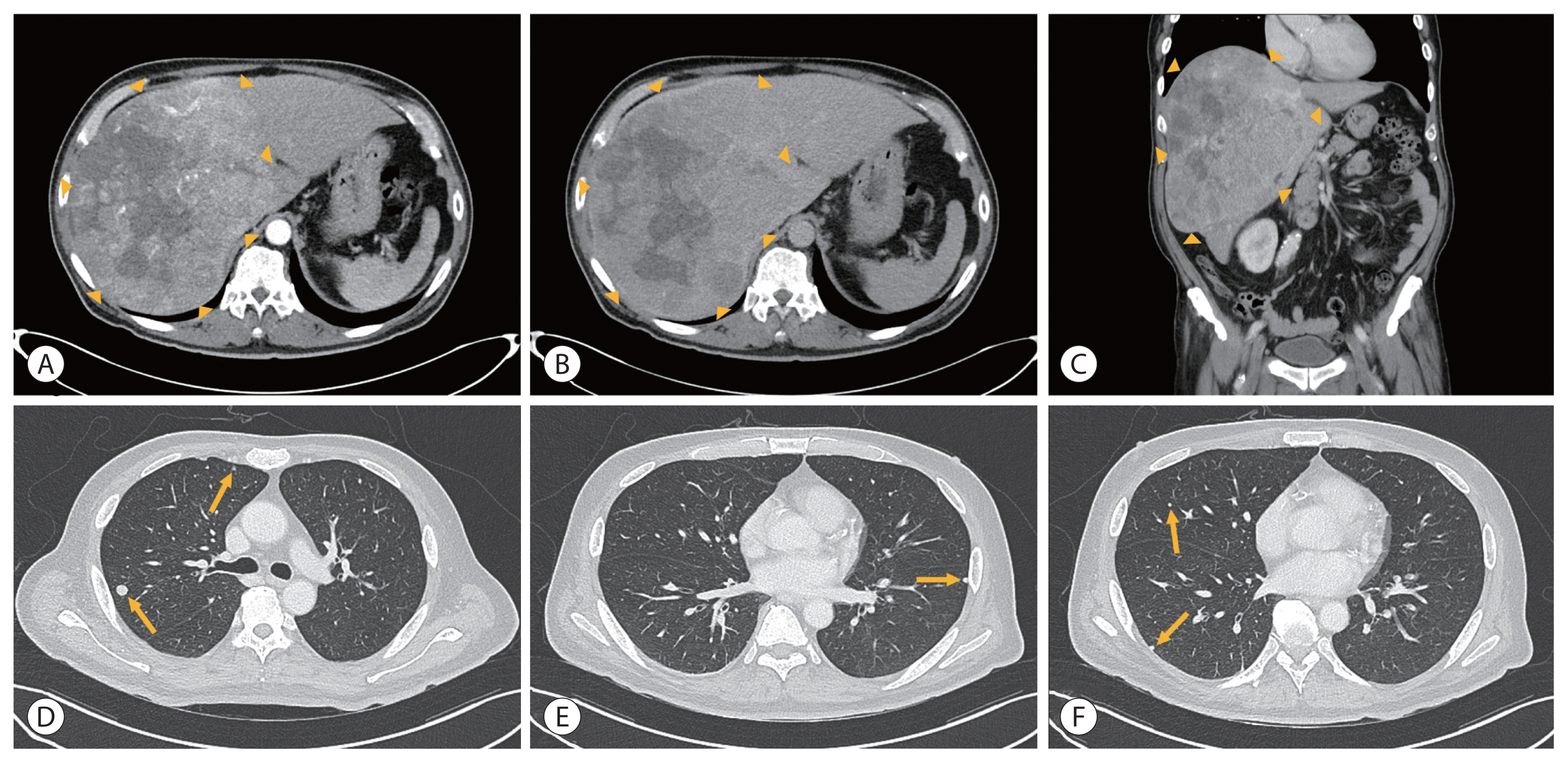 | Figure 4Initial liver dynamic computed tomography of the second patient showed approximately 23.5×13.7 cm of the heterogeneously enhanced mass in the arterial phase (A) with washout in the delayed phase (B). Coronal section in the portal phase (C). The boundaries of the tumor are indicated with arrowheads (A–C). Multiple lung metastases were observed in both lung fields (D–F, marked with arrows). 
|
Based on the history of alcohol intake and the clinical and imaging findings, the hepatic mass was diagnosed as HCC. A CT scan of the chest showed multiple metastases in both lung fields. Child-Pugh score of 5, class A and ECOG score of 0. This patient was diagnosed with mUICC stage IVb, T4N0M1, and BCLC stage C. Initial AFP was 6,528 ng/mL and PIVKA-II was 100,000 mAU/mL. Esophagogastroduodenoscopy before starting HCC treatment showed no esophageal or gastric varices.
Previous studies have found that controllability of intrahepatic tumors and hepatic reserves were significant predictors of survival in patients with advanced HCC and extrahepatic metastases, emphasizing the importance of therapeutic approaches to control intrahepatic tumors.
16,
17 We chose a treatment option with transarterial chemoembolization (TACE), because of the high tumor burden of the intrahepatic mass of the patient. In addition, we added HAIC, because previous reports showed good treatment efficacy of HAIC against advanced stage HCC with extrahepatic metastases and proved that the overall response rate of HAIC with TACE was superior to that of TACE alone.
12,
13,
18 For conventional TACE, a mixture of 10 mL iodized oil (Lipiodol; Laboratoire Guerbet, Aulnay-Sous-Bois, France) and 50 mg doxorubicin was infused into the selected feeding arteries. Embolization was performed using absorbable gelatin sponge particles (Gelfoam; Upjohn, Kalamazoo, MI, USA) cut into 1 mm
2 pieces. For HAIC, 500 mg/m
2 5-FU for 3 days and 60 mg/m
2 cisplatin for 1 day were administered at 3-week intervals.
After the 3rd cycle of TACE and the 5th cycle of HAIC, follow-up liver dynamic CT showed decreased, although remaining, arterial enhancement in the treated HCC in the right lobe, which was considered as the viable portion of the existing tumor. Additionally, follow-up chest CT revealed increased size and number of multiple lung nodules, which corresponded to disease progression according to mRECIST 1.1 (
Fig. 5). We changed the therapeutic plan to first-line systemic therapy using AteBeva. A fixed dose of 1,200 mg atezolizumab and 15 mg/kg of bevacizumab was administered at 3-week intervals. Response was evaluated after every three cycles of AteBeva.
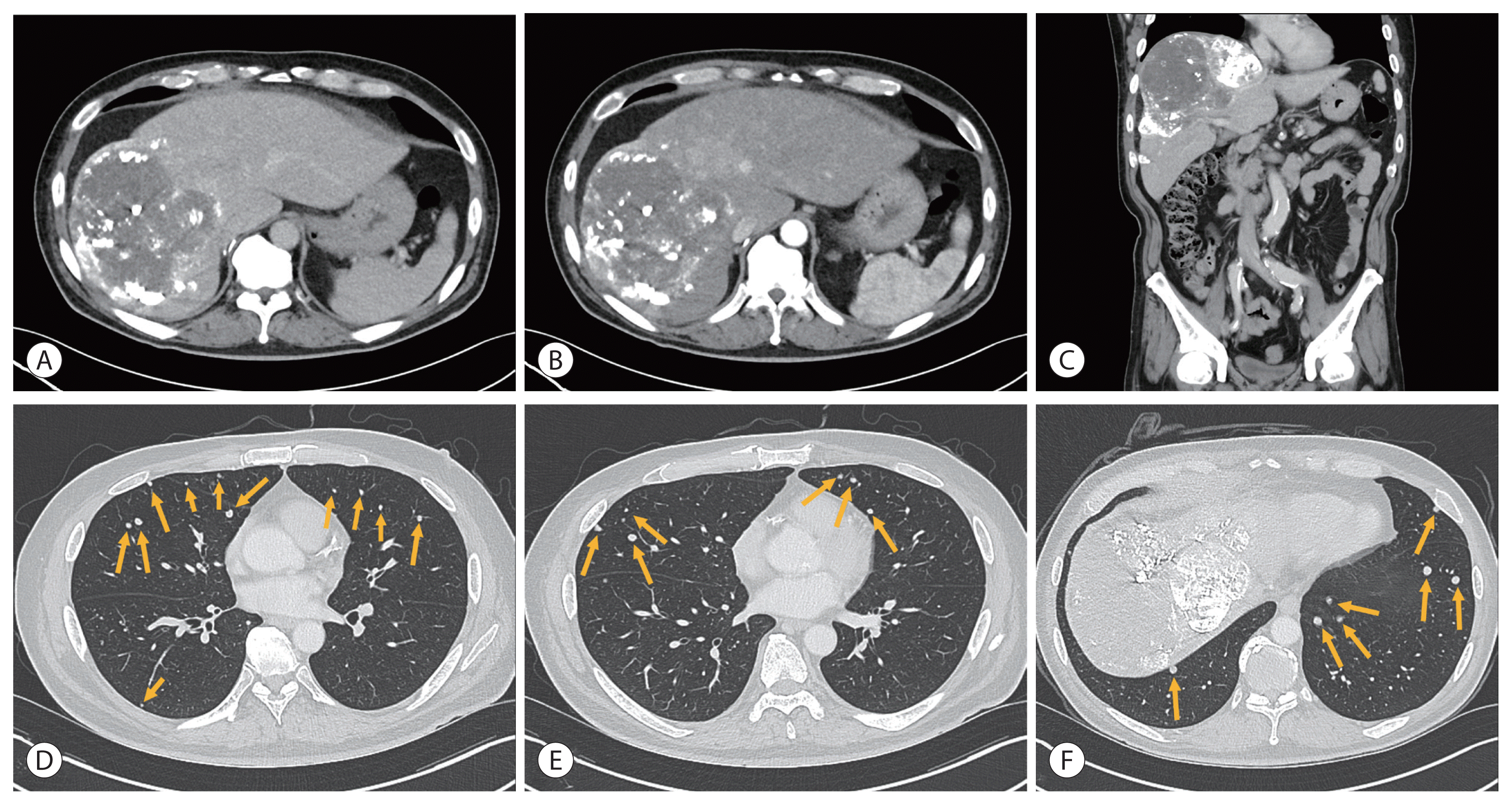 | Figure 5Follow-up liver dynamic computed tomography after the 3rd cycle of transarterial chemoembolization and the 5th cycle of hepatic arterial infusion chemotherapy showed decreased size of hepatocellular carcinoma in the arterial and delayed phase with viable area (A, B). Coronal section in the portal phase (C). Progression of lung metastases was observed in both lung fields (D–F, marked with arrows). 
|
After three doses of AteBeva, multiple lung metastases decreased according to follow-up chest CT, but two new ovoid masses less than 1.9 cm at the anterior portion of the right hepatic dome with arterial enhancement and early washout in the portal phase were observed on follow-up gadoxetic acid-enhanced liver MRI. We performed TACE three times while maintaining AteBeva, and the follow-up liver CT showed no definite viable portion on treated HCCs in the arterial phase and obtained radiological complete response for the intrahepatic mass.
However, after an additional 10 doses of AteBeva, follow-up chest CT showed increased size and number of nodules in both lungs, indicative of disease progression. The treatment regimen was changed to lenvatinib (Lenvima
®, 12 mg per day; Eisai Co., Ltd., Tokyo, Japan), which is a tyrosine kinase inhibitor (TKI). After 3 months of treatment with the new medication, follow-up chest CT showed little interval change in the size and number of lung nodules, and follow-up liver dynamic CT showed multiple lipiodol uptakes in treated HCCs without definite evidence of viable arterial enhancement. Total treatment response was defined as PR, based on mRECIST 1.1 (
Fig. 6).
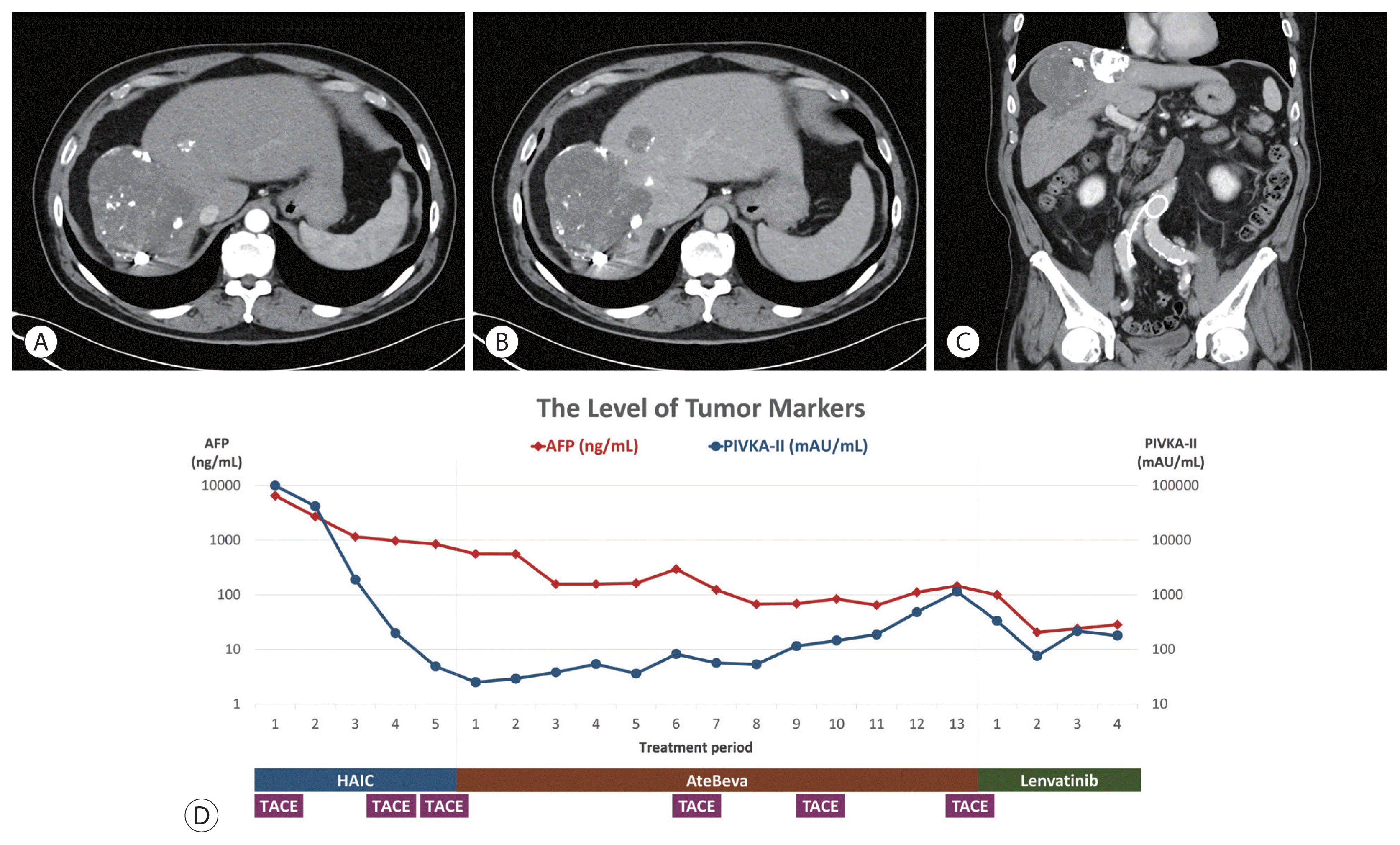 | Figure 6Follow-up liver dynamic computed tomography after treatment with lenvatinib for 3 months showed decreased size of hepatocellular carcinoma in the arterial and delayed phase without viable area (A, B). Coronal section in the portal phase (C). Changes in tumor marker levels. Y-axis is in log scale (D). AFP, alpha-fetoprotein; PIVKA-II, prothrombin-induced by vitamin K absence or antagonist-II; HAIC, hepatic arterial infusion chemotherapy; AteBeva, atezolizumab plus bevacizumab; TACE, transarterial chemoembolization. 
|
Go to :

DISCUSSION
HCC is the most common malignant tumor of the liver with a high mortality rate because most HCCs are diagnosed at an advanced stage.
10 Therefore, advanced stage HCCs have a poor prognosis and, treatment is mostly palliative.
5 Recent studies have found that immune checkpoint inhibitors (ICIs) are a promising therapeutic option for advanced stage HCC and have focused on the potential combination of ICIs with tumor microenvironment-modifying agents.
9 The IMbrave150 trial showed that the survival rate of advanced stage HCC patients was higher after treatment with atezolizumab - an antibody against programmed cell death ligand 1 (PD-L1) - plus bevacizumab, an anti-vascular endothelial growth factor (VEGF) antibody, than with sorafenib.
9 However, although anti-PD-L1 plus anti-VEGF improved treatment outcomes, approximately two-thirds of advanced stage HCC patients still do not benefit from treatment.
5 Thus, a large number of ongoing studies aim to improve the treatment outcome with ICIs alone or in combination with systemic or loco-regional therapies.
5
Our first case was a patient with stage IVa (T4N0M0) (according to mUICC), or advanced stage (according to BCLC) HCC, who had chronic hepatitis B infection. We chose a combination therapy of AteBeva every 3 weeks followed by HAIC based on 5-FU with cisplatin because our previous study showed that a pretreatment tumor-to-liver apparent diffusion coefficient (ADC) ratio of <0.741 on MRI was a predictor of an objective response to HAIC.
6 Since the pretreatment ADC ratio on MRI was 0.592, we expected a good response to HAIC. However, after the 4th cycle of AteBeva with HAIC, liver dynamic CT revealed a decreased intrahepatic mass but an extension of the right PVTT. We added RT to the remaining intrahepatic mass and PVTT because previous studies found that RT promotes localized tumor killing and potentiates the action of ICIs by modifying the tumor microenvironment.
9 After the 8th cycle of treatment, we obtained PR on intrahepatic mass and stable disease on PVTT; the total response was determined as PR.
The second case was an HCC patient who had been consuming alcohol for several years. His mUICC stage was IVb (T4N0M1) and BCLC stage was C. We chose a treatment option with TACE and HAIC to control the intrahepatic tumor. The patient’s pretreatment ADC ratio on MRI was 0.643, and a good response to HAIC was expected. However, due to progression of lung metastases after the 3rd cycle of TACE and the 5th cycle of HAIC, we changed the regimen to AteBeva for systemic therapy. This therapy improved metastatic lung lesions, however two intrahepatic viable lesions were soon discovered. We added TACE because it may elicit “immunogenic cell death” by exposing tumor antigens, thus promoting antitumor immunity.
7 The combination of ICIs with TACE is expected to enhance the antitumor response without excessive toxicity, based on recent preliminary data from single-arm studies.
7 However, because the lung metastases progressed after the 13th AteBeva administration, we changed the treatment regimen to lenvatinib. After 3 months, the total response was PR, with maintained radiological complete response in the intrahepatic lesion and little change in lung metastases. A recent multinational multicenter retrospective study demonstrated the efficacy of lenvatinib on PFS in patients with advanced HCC with progression after AteBeva treatment.
19 This report was the first to investigate second-line treatment after progression despite AteBeva treatment in HCC patients; however, the mechanisms involved in lenvatinib treatment remain unclear. Further studies are required to determine the various treatment options and the mechanism of the second-line regimen after AteBeva treatment.
Multidisciplinary HCC treatment was accompanied by several adverse events. In the first patient, renal function decline and bone marrow suppression occurred as 5-FU and cisplatin-based chemotherapy progressed. Therefore, we reduced the dose of 5-FU and cisplatin to up to 25% of the baseline. The second patient developed hypothyroidism related to atezolizumab administration. Levothyroxine was administered to replace his thyroid function.
HCC is a complex disease with various etiologies. Impaired liver function due to cirrhosis worsens prognosis and complicates clinical decisions for treatment.
2 The efficacy of many new drugs, including ICIs, has been proven for treatment against advanced stage HCC. Combination and sequential therapies with HAIC and ICIs, TKIs, anti-VEGF, and loco-regional treatments such as TACE, transarterial radioembolization, RT, and radiofrequency ablation are currently on the trials and, they are considered to have treatment potentials in highly selected patients.
2 Continuous and additional large-scale studies are required to examine treatment outcomes from various combinations and to establish the best treatment strategies. Through these efforts, many patients with advanced HCC may benefit from individualized treatment with various options.
Go to :










 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download