Abstract
Transarterial radioembolization (TARE) with yttrium 90 (90Y) has been used in the management of hepatocellular carcinoma (HCC) for more than 10 years in Korea. There are two types of 90Y radioactive microspheres available, namely, glass and resin microspheres, with comparable clinical outcomes. In general, TARE outperforms transarterial chemoembolization regarding post-embolization syndrome, time to progression, tumor downsizing for liver transplantation, and hospitalization stay. Although TARE is commonly recommended for patients with unresectable large HCCs, it can be an alternative to or performed in combination with ablation, surgical resection, and systemic treatment. This review aimed to address 90Y radioactive microspheres, patient selection, clinical outcomes, simulation tests, radioembolization procedures, follow-up imaging, and complications.
Transarterial radioembolization (TARE), also called radioembolization and selective internal radiation therapy, is a potent intra-arterial therapy that uses radioactive microspheres impregnated with yttrium 90 (90Y). TARE is considered an alternative treatment option for transarterial chemoembolization (TACE) in selected patients with hepatocellular carcinoma (HCC) in Korea and has been partly reimbursed by the National Health Insurance Service since December 2020. To date, the overall survival rates of TACE and TARE appear to be similar, but TARE is associated with a longer progression-free survival, better quality of life, and shorter hospitalization stay than TACE.1–4 This review summarizes the essential features of TARE for HCC.
Beta rays emitted from radioactive microspheres can penetrate the tissue at a mean depth of 2.5 mm and a maximum depth of up to 10 mm.5 The half-life of 90Y is approximately 2.7 days, and nearly 90% of beta rays are emitted within 7 days. The mechanism of action of TARE involves the generation of free radicals by ionization of water molecules, which causes permanent DNA damage and apoptosis of tumor cells.
Currently, two 90Y products are commercially available: TheraSphere® glass microspheres (Boston Scientific, Marlborough, MA, USA) and SIR-Spheres® resin microspheres (Sirtex Medical, Woburn, MA, USA). Because glass microspheres have a high radiation activity per sphere, fewer microspheres are needed for treatment, resulting in minimal embolic effects. Because resin microspheres have low radiation activity per sphere, a greater number of microspheres are used for treatment, resulting in even tumor coverage at the cost of a moderate embolic effect. Although both microspheres are different in terms of their composition, size, embolic effect, and specific activity per sphere (Table 1), current literature has shown comparable clinical outcomes.6–9 Theoretically, while resin microspheres are more suitable for large tumors because even tumor coverage is expected owing to the high number of microspheres, glass microspheres are more suitable for radiation segmentectomy because high radiation activity can be delivered into a segmental hepatic artery without any remarkable embolic effect. However, the application of radiation segmentectomy with resin microspheres is possible without notable embolic effects by using a 3-day pre-calibration dose. For large tumors, glass microspheres can have a sufficient number of microspheres by using multiple vials of the second-week dose. Thus, the authors do not have any preference in daily clinical practice and commonly choose microspheres that can be delivered earlier.
The indications for TARE largely overlap with those for TACE in terms of tumor stage. However, given the high cost of TARE, the patients’ economic status is the biggest hurdle in selecting this treatment. Although TARE is now partly reimbursed by the National Health Insurance Service in Korea, it still costs 7–10 times more than TACE. Patients have to pay KRW 200,000–800,000 (approximately USD 170–700) for TACE and KRW 8,000,000 (approximately USD 7,000) for TARE.
Because TARE is more potent than TACE in terms of damage to the healthy liver as well as tumor control, patients should have good liver function of Child-Pugh class A. Therefore, the most favored candidates for TARE are patients with an unresectable HCC who have Child-Pugh class A. The biggest advantage of TARE over TACE is that it induces minimal post-embolization syndrome irrespective of the tumor size, whereas post-embolization syndrome after TACE is commonly proportional to the tumor burden. Thus, TARE is commonly considered for patients with a large (>6 cm) unresectable HCC who can benefit more from TARE than for those with a small HCC.
Regarding contraindications, patients with an Eastern Cooperative Oncology Group score of >2 or Child-Pugh class of ≥B8 are relatively contraindicated. Patients with a serum bilirubin level of ≥2 mg/dl, tumor burden >70% of liver volume, and cirrhotomimetic-type HCC are also commonly excluded.4
In general, TARE is safe for tumors abutting the stomach, duodenum, and colon because normal peristalsis can prevent excessive irradiation to the bowel, especially at the same spot from the tumor. However, in patients who had a previous abdominal surgery, adhesion between the bowel and tumor may cause inadvertent radiation damage to the bowel.
Theoretically, TARE can be a good treatment option for patients with portal vein tumor invasion because of its minimal embolic effect. However, in these patients, an arterioportal shunt is commonly present. Because of the small particulate sizes, both 99m-Technetium-tagged macroaggregated albumin (99mTc-MAA) and radioactive microspheres can flow into the portal vein through the arterioportal shunt, which can be observed on single-photon emission computed tomography (SPECT)/computed tomography (CT) and positron emission tomography (PET)/CT, respectively. When the arterioportal shunt is limited to the target lobe, TARE can be safely performed in most cases.10 However, when the arterioportal shunt reaches the contralateral lobar portal vein, it is a relative contraindication for TARE. On CT and magnetic resonance imaging (MRI) of the hepatic arterial phase, intense contrast enhancement of the portal vein indicates the presence of an arterioportal shunt. If the main portal vein or contralateral lobar portal vein is enhanced with a similar degree of the aorta on CT/MRI in the arterial phase, then patients should be contraindicated (Fig. 1).
TARE can also be performed in patients with intrahepatic cholangiocarcinoma or metastatic liver tumors11 because TARE is expected to be effective for hypervascular tumors, regardless of the tumor type. In the authors’ institute, patients with liver metastasis or cholangiocarcinoma are sporadically referred for TARE when single or oligonodular tumors are resistant to standard chemotherapy and unresectable because of anatomical or clinical reasons. If tumors are hypervascular on angiography and selective TARE appears to be technically feasible, then TARE can be a useful option in these patients.
Some patients with HCC are inoperable because of a small future liver remnant (FLR). Although portal vein embolization (PVE) is an established method to increase the FLR volume to more than 40% before resection, PVE necessitates 1–1.5 months of delay of surgery, which may incite progression of an untreated tumor during that period. On the contrary, radiation lobectomy, which involves lobar infusion of 90Y, induces a volumetric increase of FLR comparable to that in PVE while controlling the tumor during the time of hypertrophy (Fig. 2).12 One disadvantage of radiation lobectomy is the longer time (3–4 months) to hypertrophy than the time taken in PVE. Given that radiation lobectomy can control ipsilateral tumors and test the tumor nature, it would be a suitable option for patients with rapidly growing HCCs.
Current literature regarding TARE of HCC has reported consistent outcomes in overall survival, from 24.4 to 26.9 months in Barcelona Clinic Liver Cancer (BCLC) stage A, from 16.4 to 18 months in BCLC stage B, and from 7.3 to 13 months in BCLC stage C.6–9
In early HCC (BCLC stage A), radiation segmentectomy (ablative radiation dose in one or two segments) is associated with better tumor response than TACE.13 A recent multicenter retrospective study showed a complete response rate of 84% for a single tumor sized <8 cm.14 Thus, in early HCC, when surgical resection or ablation is not feasible, TARE is suggested as an alternative option with a curative intent.
Unlike most studies that failed to show differences in overall survival between TARE and TACE in BCLC stage B, a few studies have demonstrated that TARE showed a longer time to progression than TACE.2,15 Therefore, TARE can play a role as a bridge to transplantation for patients with a long waiting time.16 TARE was also more effective in downstaging tumors of the United Network for Organ Sharing T3 to T2 than TACE. TARE can be performed on an outpatient basis owing to the minimal incidence of post-embolization syndrome, whereas TACE generally requires a hospitalization period that is largely proportional to the tumor size for the management of post-embolization syndrome. Given that most studies have consistently demonstrated that TARE yields shorter hospitalization times, fewer treatment sessions, and fewer hospital visits than TACE, TARE is superior to TACE in terms of quality of life.3
In BCLC stage C, systemic therapy such as sorafenib or atezolizumab/bevacizumab is regarded as the standard of care. Recent global randomized trials showed comparable outcomes between TARE and sorafenib for locally advanced disease. 17,18 Although the combination of TARE and sorafenib showed a similar outcome as that with sorafenib alone,19 combination therapy of TARE followed by an immune-oncologic drug seems to be promising.20 However, TACE followed by external radiation therapy is commonly performed in patients with portal vein tumor thrombus in Korea, and its performance was proved in a single-center randomized study.21 Thus, a randomized study comparing TARE and TACE followed by external radiation therapy is needed. Because TARE is a locoregional therapy, the extent of portal vein tumor thrombus is an important prognostic factor. In patients with lobar or main portal vein tumor thrombus, the median overall survival was reported as 7.7–14.2 months.22,23 In patients with segmental portal vein tumor thrombus, the median overall survival was reported to be up to 28 months.22
TARE generally requires a pretreatment simulation test, including hepatic angiography, 99mTc-MAA injection to the target arteries in an interventional suite, and 99mTc scintigraphy (planar and SPECT/CT scans) to measure the lung shunt fraction (LSF).
Angiographic evaluation should be performed to scrutinize the celiac trunk and hepatic artery anatomy, non-hepatic artery from the hepatic artery, possible extrahepatic collateral arteries, portal vein patency, and presence of arterioportal shunting. Coil embolization of the gastroduodenal artery to prevent inadvertent administration of 90Y is no longer routinely recommended, 24 and nowadays, the authors do not perform embolization of the gastroduodenal artery. Non-hepatic arteries originating from the hepatic artery (e.g., accessory left gastric arteries, right gastric arteries, hepatic falciform arteries, and esophageal branches from the replaced left hepatic artery) are commonly embolized before 99mTc-MAA injection when needed.
Radioactive microspheres (size: 20–60 μm) were sufficiently large to not pass through the liver sinusoid. Thus, LSF is almost always less than 5% in patients with an HCC of size <5 cm, which means that the simulation test may be skipped in patients with small HCCs (<5 cm).25 However, large HCCs commonly have dilated leaky intratumoral vessels, and radioactive microspheres may pass through the hepatic venous drainage and then be captured in the pulmonary arterioles, resulting in radiation pneumonitis.26 Because radiation pneumonitis can be fatal, LSF must be investigated before performing radioembolization in patients with an HCC of >5 cm. In the authors’ institute, the simulation test is mandatory in patients with an HCC of >4 cm and is occasionally skipped in patients with an HCC of <4 cm.
99mTc-MAA with a size range of 10–90 μm, a surrogate of radioactive microspheres of similar size, is injected into the hepatic artery, and the LSF is commonly calculated based on planar scan images. For SIR-Spheres®, 20% of LSF is a suggested limit, and reduced activity is recommended for patients with 10–20% of LSF, when body surface area dosimetry is used.27 In Asia, however, partition dosimetry is commonly used for SIR-Spheres®, and a lung radiation dose of >25 Gy is considered as the upper limit. For TheraSphere®, a radiation dose of >30 Gy to the lung per treatment or a cumulative dose of 50 Gy to the lung is the limit according to medical internal radiation dose dosimetry. Notably, the radiation dose to the lung depends on the target tissue volume as well as LSF. For example, TARE can be performed in patients with a small HCC and 20% LSF, but TARE could be very risky in patients with a large (>15 cm) HCC and 15% LSF. Thus, the absolute value of LSF does not determine the feasibility of TARE.
Although SPECT/CT is not essential in dosimetry, it can depict the exact distribution of 99mTc-MAA on cross-sectional images, which enables the prediction of tumor responses and elaboration of dosimetry based on a multi-compartment model. In a recent trial, TARE with personalized dosimetry improved overall survival.28 In addition, extrahepatic deposition of 99mTc-MAA can be occasionally detected on SPECT images, which can be useful for operators to prevent non-target irradiation by embolizing the non-target vessels or adjusting the microcatheter position during the main procedure.
Because of the complexity of TARE simulation and planning, collaboration between interventional radiology and nuclear medicine is important. Handling radioisotopes including 99mTc-MAA and 90Y microspheres, radioactive waste disposal, and dispensing resin microspheres are managed by nuclear medicine technicians. A nuclear medicine physician calculates the lung shunt fraction and personalized dosimetry using a multi-compartment model.
TARE is commonly performed 1–2 weeks after the simulation test. The procedure is quite similar to TACE, except for the use of radioactive microspheres. Unlike conventional TACE, radioactive microspheres are radiolucent and the operator cannot see the distribution of radioactive microspheres on fluoroscopy. Radioactive microspheres are commonly infused at the lobar or segmental hepatic artery with antegrade blood flow, and their distribution is proportional to the blood flow. Fortunately, most HCCs are hypervascular, and the density of microspheres in HCCs is much higher than that in the normal liver. Thus, excessive superselective catheterization (i.e., wedged catheter position) is neither desirable nor required in most cases.
Patients with high LSF, usually having a huge tumor or hepatic vein invasion, can be managed by TARE after several methods to prevent radiation pneumonitis. The safest method is to choose an alternative treatment such as TACE or systemic therapy. Systemic therapy such as sorafenib may reduce LSF, and LSF can be reassessed 1 or 2 months after systemic therapy. However, unless systemic therapy has sufficient antitumor effects, a reduction in LSF cannot be expected. Shunt reduction procedures, such as hepatic vein ballooning and periprocedural bland embolization, have shown positive results. However, this procedure is not widely performed because the actual LSF after the reduction procedure cannot be measured on-site and because patients do not need to risk radiation pneumonitis in general. In our institute, for high LSF, conventional TACE or bland embolization followed by TARE is recommended as the first option for patients with locally advanced HCC without metastasis.29
PET and SPECT can be used for post-procedural imaging to investigate the actual distribution of radioactive microspheres. As Bremsstrahlung photons are produced because of the interaction of the beta ray with the tissue, SPECT can image the 90Y microspheres in the liver. However, the clinical utility of SPECT is limited because of its low image resolution. By contrast, 90Y PET can present 90Y images with a higher spatial resolution. Although 90Y microspheres emit a very small number of positrons (1/700 than Bremsstrahlung photons), 90Y PET is superior to Bremsstrahlung SPECT for the assessment of microsphere distribution.30 The high resolution of 90Y PET/CT enables the assessment of extrahepatic deposition of 90Y microspheres as well as intratumoral accumulation, suggesting that both tumor response and possible complications are predictable. Unfortunately, 90Y PET is not reimbursed by the National Health Insurance Service in Korea.
Because the penetration depth of beta rays into the tissue is less than 1 cm, patients who undergo radioembolization do not need to be isolated. Family members can live with patients without radiation risk.31 However, it is not recommended for children to stay in close proximity for 7 days.
Surgical resection is not recommended within 30 days of the procedure because the operator may be exposed to beta rays. However, if surgery is needed within 30 days, then special handling is required, including the use of lead gloves, special instruments, or extremity radiation monitoring equipment such as a ring badge.27 The surgical specimen should be stored in a leak-proof container within 2 months after the procedure and be placed behind lead shielding for decay during storage. Pathological analysis of the specimen becomes feasible after 60 days of decay.
In contrast to TACE treatment, HCC treated with TARE shows a slow decrease in tumor size and enhancement. Therefore, on early follow-up imaging (1–3 months after TARE), persistent tumoral enhancement without size reduction (stable disease by modified Response Evaluation Criteria in Solid Tumors [mRECIST]) is quite common, but it does not mean incomplete treatment.32 Complete disappearance of tumor enhancement may take several months after TARE (Fig. 2), and patients can be observed without additional treatment for 1 year unless tumor markers show rebound or new lesions are observed on imaging. Although boosted TARE (ablative dose of TARE such as radiation segmentectomy) may cause rapid disappearance of tumor enhancement within 3 months,33 tumor markers can be more sensitive than imaging studies in most cases in terms of early (3–6 months) tumor response assessment. However, caution is needed in the interpretation of tumor markers because patients with normal levels of baseline alpha-fetoprotein (AFP) occasionally have mild elevation of AFP to approximately 30–100 ng/mL in the early follow-up period owing to normal liver damage by TARE.
Radiation necrosis of the normal hepatic parenchyma can occasionally be observed in superselective TARE and is shown as a newly developed hypovascular lesion mimicking hypovascular HCC. 90Y PET/CT may be useful for differentiating radiation necrosis from a hypovascular tumor (Fig. 3).
Peritumoral ring enhancement may persist for several months, mimicking marginal recurrence or a peripheral residual tumor. Peritumoral ring enhancement corresponds to granulation tissue and the fibrous pseudocapsule surrounding the tumor on pathological examination. Peritumoral ring enhancement usually shows circumferential enhancement with even thickness without washout in the portal venous and delayed phases, whereas a marginal recurrence presents nodular enhancement with washout.
In general, additional treatment is recommended when definite progressive disease on imaging studies (increased enhancing part of the tumor or new lesion) is noted or when tumor markers show rebound. In the authors’ institute, as boosted TARE is commonly performed, additional treatment is recommended when residual tumoral enhancement is identified on a 6-month imaging study.
Minimal post-embolization syndrome and related advantages (e.g., possibility of outpatient-based treatment and quality of life) are the most attractive aspects of TARE. By contrast, TARE can be a risky treatment option unless non-target irradiation that necessitates surgical management is prevented by comprehensive angiographic evaluation and the anatomy knowledge of operators.
The cystic artery can originate from anywhere between the main trunk of the right hepatic artery to segmental hepatic arteries. Although the incidence of radiation cholecystitis requiring surgical cholecystectomy is low, radiological abnormalities, such as gallbladder wall hyperenhancement, gallbladder wall edema, and mural disruption, are commonly observed after TARE. Radiation cholecystitis may develop 1–3 months after the procedure and commonly presents as abdominal pain. Surgical cholecystectomy is needed for pain intractable to medical treatments such as analgesics and percutaneous drainage.
Benign biliary stricture is rare but is reported more commonly in patients with infusion of radioactive microspheres into the caudate artery.34 Once the stricture develops, symptomatic cholangitis and jaundice are commonly observed.
Unintended infusion of 90Y microspheres into the accessory left gastric, right gastric, and gastroduodenal arteries incites radiation ulcers in the stomach and duodenum. Radiation ulcers may present with abdominal pain and gastrointestinal hemorrhage and generally necessitate surgical resection.35
Radiation pneumonitis is uncommon and requires proper dosimetry following a lung shunt study. When the right inferior phrenic artery supplies the HCC, administration of 90Y microspheres to the artery should be carefully determined. Because the right inferior phrenic artery accompanies pulmonary shunting in many cases, angiographic evaluation and knowledge of dangerous arterial branches are required to treat the artery safely.36 Non-productive cough and dyspnea are common symptoms of radiation pneumonitis, which may occur 1–6 months after the procedure.26 Steroid treatment is effective in many cases, but radiation pneumonitis can be fatal in serious cases. CT scans show consolidation and ground-glass opacity with sparing of the subpleural area, which is the so-called bat wing appearance.
The typical manifestation of radioembolization-induced liver disease consists of anicteric ascites, increased alkaline phosphatase levels, and thrombocytopenia. The exact tolerable dose to the liver after TARE remains unknown. Furthermore, the true absorbed dose to the normal liver can be difficult to assess. In this regard, the easiest and most effective way to avoid radioembolization-induced liver disease is to avoid whole-liver irradiation, regardless of whether it is a single or sequential treatment.
TARE is a potent but expensive intra-arterial treatment with minimal post-embolization syndrome for patients with HCC. It can offer a curative chance for early HCC, effective tumor control, bridging/downstaging for intermediate HCC, and comfortable palliation for advanced HCC. TARE can be actively recommended regardless of the tumor stage if a patient with Child-Pugh class A can afford it financially.
Notes
Ethics Statement
This review article is fully based on the articles which was already published and did not involve additional patient participants. Therefore, IRB approval is not necessary.
References
1. Kim HC. Radioembolization for the treatment of hepatocellular carcinoma. Clin Mol Hepatol. 2017; 23:109–114.

2. Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu RK, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011; 140:497–507.

3. Salem R, Gilbertsen M, Butt Z, Memon K, Vouche M, Hickey R, et al. Increased quality of life among hepatocellular carcinoma patients treated with radioembolization, compared with chemoembolization. Clin Gastroenterol Hepatol. 2013; 11:1358–1365.

4. Kallini JR, Gabr A, Salem R, Lewandowski RJ. Transarterial radioembolization with yttrium-90 for the treatment of hepatocellular carcinoma. Adv Ther. 2016; 33:699–714.

5. Salem R, Lewandowski RJ, Sato KT, Atassi B, Ryu RK, Ibrahim S, et al. Technical aspects of radioembolization with 90Y microspheres. Tech Vasc Interv Radiol. 2007; 10:12–29.
6. Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, et al. Radioembolization for hepatocellular carcinoma using yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010; 138:52–64.

7. Hilgard P, Hamami M, Fouly AE, Scherag A, Müller S, Ertle J, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010; 52:1741–1749.

8. Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011; 54:868–878.

9. Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013; 57:1826–1837.

10. Thornton LM, Geller BS, Pepin EW, Shah JL, Kapp M, Toskich BB. Safety of radioembolization in the setting of angiographically apparent arterioportal shunting. J Vasc Interv Radiol. 2018; 29:1511–1518.

11. Lee EJ, Chung HW, Jo JH, So Y. Radioembolization for the treatment of primary and metastatic liver cancers. Nucl Med Mol Imaging. 2019; 53:367–373.

12. Vouche M, Lewandowski RJ, Atassi R, Memon K, Gates VL, Ryu RK, et al. Radiation lobectomy: time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J Hepatol. 2013; 59:1029–1036.

13. Padia SA, Johnson GE, Horton KJ, Ingraham CR, Kogut MJ, Kwan S, et al. Segmental yttrium-90 radioembolization versus segmental chemoembolization for localized hepatocellular carcinoma: results of a single-center, retrospective, propensity score-matched study. J Vasc Interv Radiol. 2017; 28:777–785.

14. Salem R, Johnson GE, Kim E, Riaz A, Bishay V, Boucher E, et al. Yttrium-90 radioembolization for the treatment of solitary, unresectable HCC: the legacy study. Hepatology. 2021; 74:2342–2352.

15. Salem R, Gordon AC, Mouli S, Hickey R, Kallini J, Gabr A, et al. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2016; 151:1155–1163.

16. Gabr A, Kulik L, Mouli S, Riaz A, Ali R, Desai K, et al. Liver transplantation following yttrium-90 radioembolization: 15-year experience in 207-patient cohort. Hepatology. 2021; 73:998–1010.

17. Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux GP, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017; 18:1624–1636.
18. Chow PKH, Gandhi M, Tan SB, Khin MW, Khasbazar A, Ong J, et al. SIRveNIB: selective internal radiation therapy versus sorafenib in asia-pacific patients with hepatocellular carcinoma. J Clin Oncol. 2018; 36:1913–1921.
19. Ricke J, Klümpen HJ, Amthauer H, Bargellini I, Bartenstein P, de Toni EN, et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol. 2019; 71:1164–1174.

20. Tai D, Loke K, Gogna A, Kaya NA, Tan SH, Hennedige T, et al. Radioembolisation with Y90-resin microspheres followed by nivolumab for advanced hepatocellular carcinoma (CA 209–678): a single arm, single centre, phase 2 trial. Lancet Gastroenterol Hepatol. 2021; 6:1025–1035.

21. Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol. 2018; 4:661–669.

22. Spreafico C, Sposito C, Vaiani M, Cascella T, Bhoori S, Morosi C, et al. Development of a prognostic score to predict response to yttrium-90 radioembolization for hepatocellular carcinoma with portal vein invasion. J Hepatol. 2018; 68:724–732.

23. Abouchaleh N, Gabr A, Ali R, Al Asadi A, Mora RA, Kallini JR, et al.
90Y radioembolization for locally advanced hepatocellular carcinoma with portal vein thrombosis: long-term outcomes in a 185-patient cohort. J Nucl Med. 2018; 59:1042–1048.

24. Hamoui N, Minocha J, Memon K, Sato K, Ryu R, Salem R, et al. Prophylactic embolization of the gastroduodenal and right gastric arteries is not routinely necessary before radioembolization with glass microspheres. J Vasc Interv Radiol. 2013; 24:1743–1745.

25. Gabr A, Ranganathan S, Mouli SK, Riaz A, Gates VL, Kulik L, et al. Streamlining radioembolization in UNOS T1/T2 hepatocellular carcinoma by eliminating lung shunt estimation. J Hepatol. 2020; 72:1151–1158.

26. Wright CL, Werner JD, Tran JM, Gates VL, Rikabi AA, Shah MH, et al. Radiation pneumonitis following yttrium-90 radioembolization: case report and literature review. J Vasc Interv Radiol. 2012; 23:669–674.

27. Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: technical and methodologic considerations. J Vasc Interv Radiol. 2006; 17:1251–1278.

28. Garin E, Tselikas L, Guiu B, Chalaye J, Edeline J, de Baere T, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol. 2021; 6:17–29.

29. Kim HC, Choi JW, Lee M, Kim YJ, Paeng JC, Chung JW. Lung shunt reduction for yttrium-90 radioembolization: chemoembolization versus radioembolization. In Vivo. 2021; 35:2305–2312.

30. Tafti BA, Padia SA. Dosimetry of Y-90 microspheres utilizing Tc-99 m SPECT and Y-90 PET. Semin Nucl Med. 2019; 49:211–217.
31. McCann JW, Larkin AM, Martino LJ, Eschelman DJ, Gonsalves CF, Brown DB. Radiation emission from patients treated with selective hepatic radioembolization using yttrium-90 microspheres: are contact restrictions necessary? J Vasc Interv Radiol. 2012; 23:661–667.

32. Joo I, Kim HC, Kim GM, Paeng JC. Imaging evaluation following 90Y radioembolization of liver tumors: what radiologists should know. Korean J Radiol. 2018; 19:209–222.

33. Kim HC, Kim YJ, Lee JH, Suh KS, Chung JW. Feasibility of boosted radioembolization for hepatocellular carcinoma larger than 5 cm. J Vasc Interv Radiol. 2019; 30:1–8.

34. Kim HC, Joo I, Lee M, Chung JW. Benign biliary stricture after yttrium-90 radioembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2020; 31:2014–2021.

Figure 1
An infiltrative hepatocellular carcinoma (HCC) in the right lobe of a 68-year-old man. Hepatitis B core antibody was positive, and Child-Pugh class was A5. His alpha fetoprotein level level was 112,600 ng/mL. (A) Computed tomography (CT) scan of the hepatic arterial phase showing an infiltrative HCC (star). Note the intense contrast enhancement of the hepatic artery (arrowhead) and the portal vein (arrow). (B) Hepatic angiogram showing a severe arterioportal shunt. The main portal vein (arrowhead) is filled with the contrast medium.
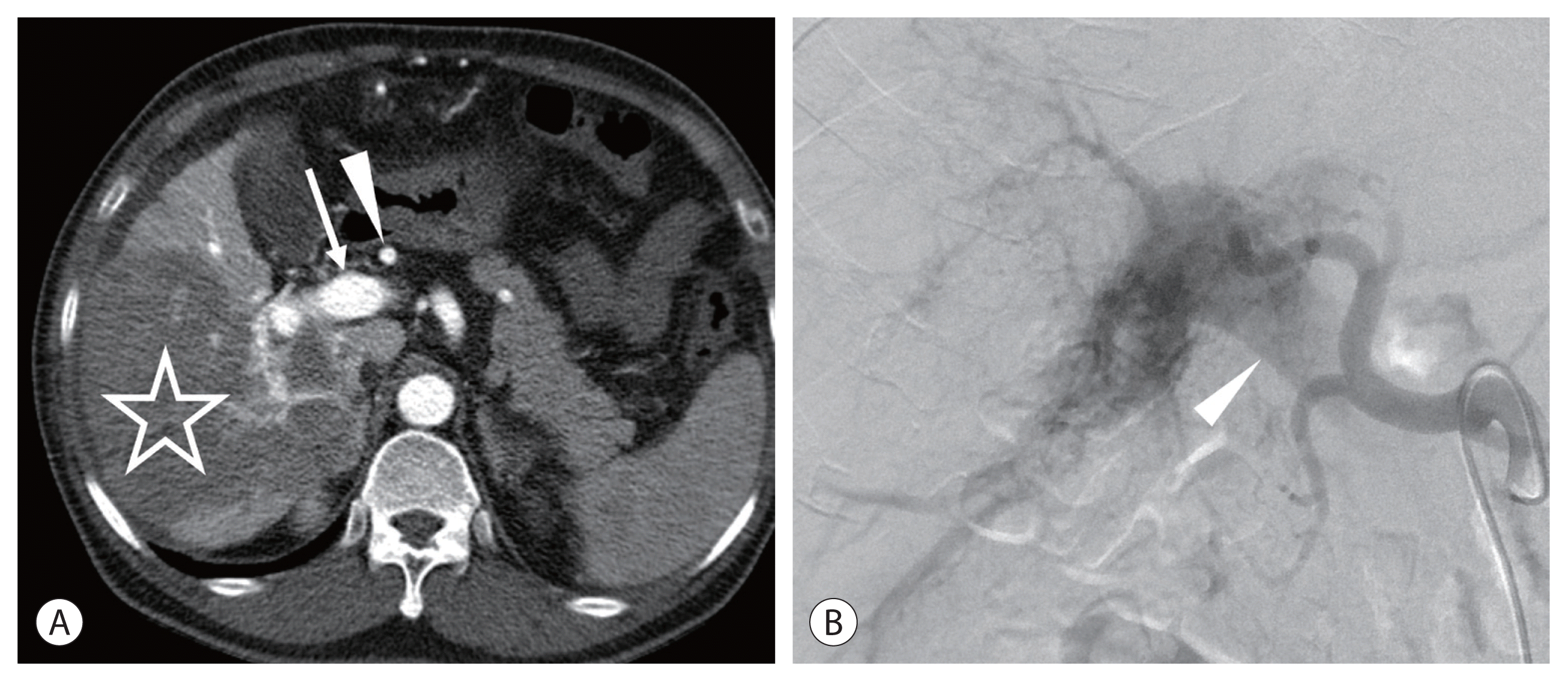
Figure 2
A 12-cm hepatocellular carcinoma (HCC) in the right lobe of a 69-year-old woman. The Child-Pugh class was A5. His alpha fetoprotein level was 29,360 ng/mL, and PIVKA-II was 2,129 mAU/mL. (A) Magnetic resonance image (MRI) showing the large HCC (arrowheads) in the right lobe. (B) Computed tomography (CT) scan taken 1 month after radioembolization showing slightly decreased tumor size (10 cm) (arrowheads) with persistent tumoral enhancement. (C) MRI taken 5 months after radioembolization showing complete disappearance of tumoral enhancement and decreased tumor size (4 cm) (arrowheads). Note the hypertrophy of the left lobe and atrophy of the right lobe of the liver.
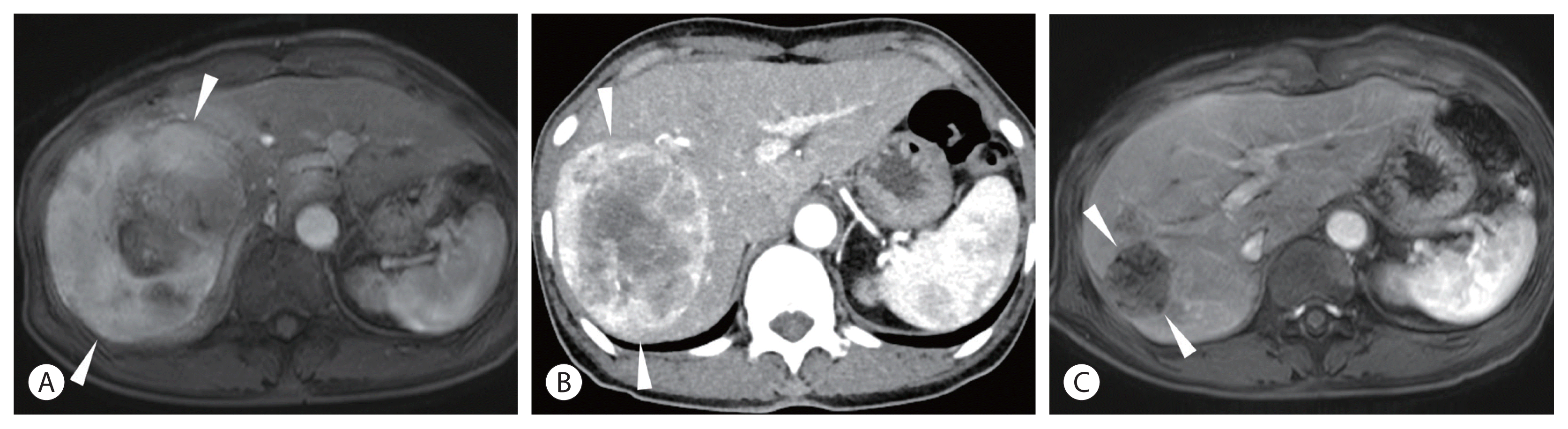
Figure 3
A 67-year-old man had a 7-cm hepatocellular carcinoma (HCC) in the right lobe. He was positive for hepatitis B surface antigen (HBsAg), and Child-Pugh class was A5. His alpha fetoprotein level was 3.4 ng/mL, and PIVKA-II was 599 mAU/mL. (A) Computed tomography (CT) scan showing the 7-cm HCC (arrowheads) in the right lobe. A small arterioportal shunt (arrow) is also noted. (B) Positron emission tomography (PET)/CT performed at the next radioembolization showing high activity in the tumor (arrowheads) as well as in the arterioportal shunt (arrow). (C) CT scan taken 5 months after radioembolization showing nearly complete disappearance of tumoral enhancement (arrowheads). A new hypovascular lesion (arrow) is seen at the same location of the arterioportal shunt, which is thought to be radiation-induced necrosis.
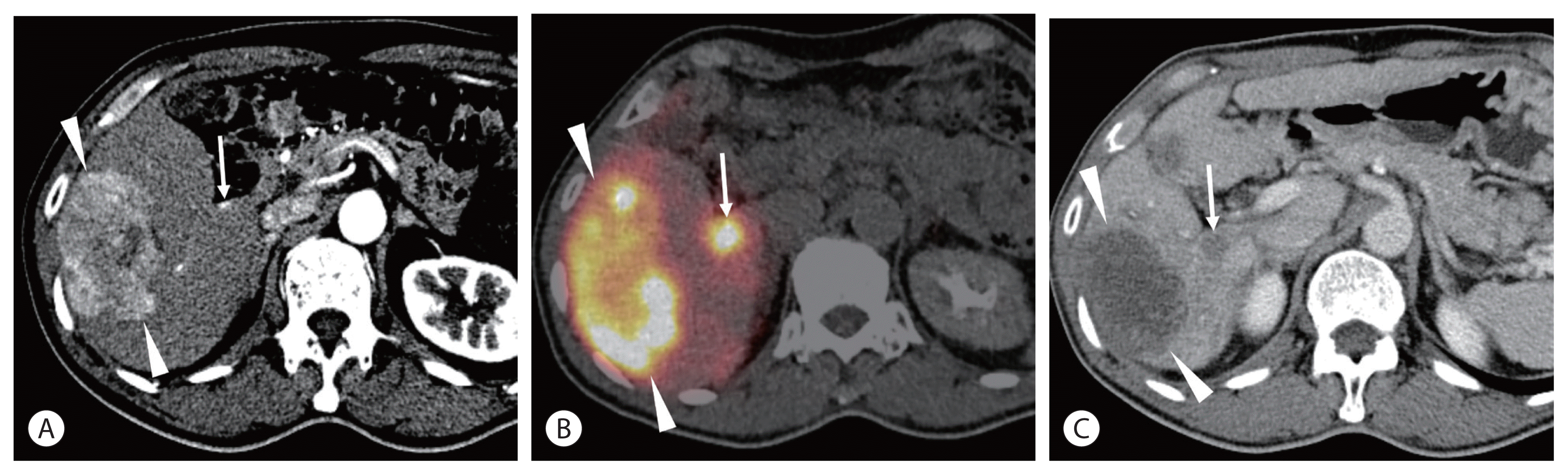
Table 1
Comparison of characteristics between glass microspheres and resin microspheres
| TheraSphere® | SIR-Spheres® | |
|---|---|---|
| Half-life | 64.2 hours | 64.2 hours |
| Material | Glass | Resin |
| Size | 20–30 | 20–60 |
| Distribution of 90Y | Mixed with glass matrix | Surface of resin sphere |
| Activity per sphere | 2,500 Bq* | 50 Gq† |
| Available vial | 3, 5, 7, 10, 15, 20 GBq* | 3–7 GBq† |
| Limitation of total activity per treatment | No | 7 GBq |
| Specific gravity | 3.6 | 1.6 |
| Number of spheres | 1.2–8 million | 40–80 million |
| Handling for dispensing | Not required | Required |
| Embolic effect | Minimal | Moderate |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download