Abstract
Purpose
Cryptoglandular fistula is one of the common anal diseases requiring surgical treatment. Various surgical techniques have been introduced; however, there is no known standard technique. Coring-out fistulectomy is a surgical technique that accurately resects only the fistula tract. However, only a few cases of this procedure have been reported. We aimed to analyze the surgical outcomes of coring-out fistulectomy for cryptoglandular anal fistulas.
Methods
We retrospectively reviewed the medical records of patients who underwent coring-out fistulectomy for a cryptoglandular fistula between 1999 and 2019. Primary outcomes were the treatment success rate (recurrence and healing rates) and incidence of fecal incontinence.
Results
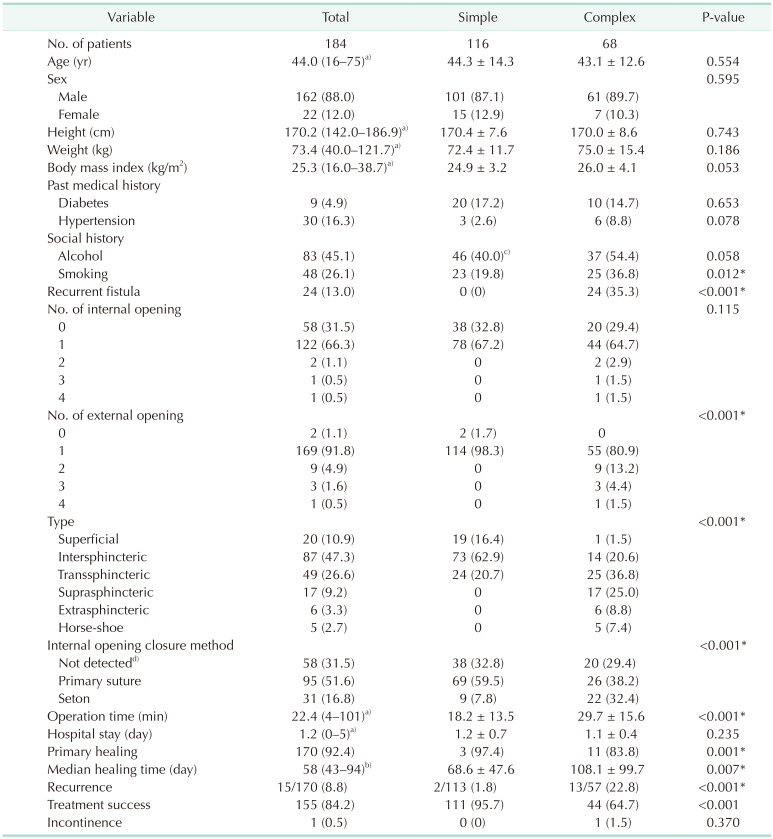
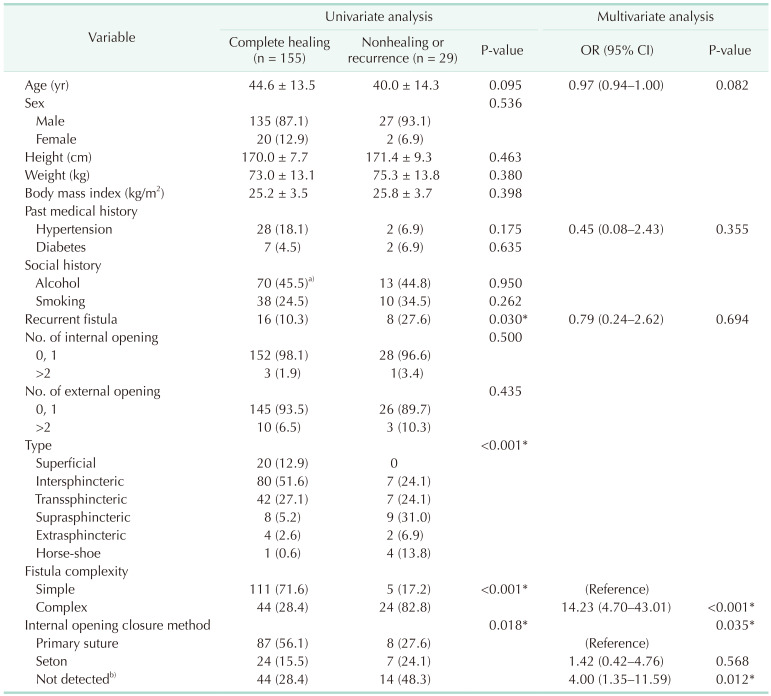
A total of 184 patients were included in our study. The average age of the patients was 44 years (range, 16–75 years), and 88.0% were male. Twenty-four (13.0%), 13 (7.1%), and 68 patients (37.0%) underwent operation for recurrent fistula, multiple tracts, and complex type fistula, respectively. The healing rate was 92.4%, and recurrence occurred in 15 of 170 healed patients (8.8%). Thus, the treatment success rate was 84.2%. There was no fecal incontinence except in 1 patient who had preoperative fecal incontinence because of cauda equine syndrome. In multivariable analysis of the factors affecting the treatment success rate, the complex fistula (odds ratio [OR], 14.2; 95% confidence interval [CI], 4.7–43.0; P < 0.001) and undetected internal opening during the operation (OR, 4.0; 95% CI, 1.4–11.6; P = 0.012) were significant factors.
Anal fistula is one of the most common anal diseases with a prevalence of 2 cases per 10,000 people/year [1]. There are several causes of anal fistula, but 90% of the cases have cryptoglandular origin [2]. In this disease, the anal crypt is blocked by inspissated debris or stool causing an infection in the anal gland, thereby forming abscess and fistula [3].
To date, various methods of surgery for anal fistulas have been introduced. The most common procedures are fistulotomy and fistulectomy [4]. Although these procedures can be performed easily and conveniently, they have the disadvantage of having a high possibility of causing fecal incontinence due to sphincter injury [5]. In particular, in the case of extrasphincteric type fistulas, up to 83% of the patients have been reported to experience incontinence. Therefore, various procedures such as sphincter-preserving surgery are performed to prevent incontinence. One of these is ligation of intersphincteric fistula tract (LIFT), a procedure introduced by Rojanasakul [6] in 2009. A recently published meta-analysis [7] reported that the overall success rate of this procedure was 76.5% and that the rate of complications was 13.9%. Video-assisted anal fistula treatment is another sphincter-preserving technique that was introduced by Meinero and Mori [8]. The recurrence rate following this procedure is reportedly 17.7% and the complication rate is 4.8% [9]. However, the reported success rates of these techniques are inconsistent, and their long-term outcomes have not been sufficiently studied.
Coring-out fistulectomy is a type of sphincter-preserving procedure that enables accurate resection of the fistula tract alone and thus reduces the possibility of missing a secondary tract. [10] This technique was first described by A. Lewis [10] who reported that fistula recurrence was noted only in 2 of the 100 patients with low fistula who underwent coring-out fistulectomy between 1972 and 1986. However, only a few cases of this procedure have been reported in the literature [111213]. Therefore, we aimed to report the surgical outcomes of coring-out fistulectomy for cryptoglandular fistula at a single center over a period of 20 years.
This study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital and was performed in accordance with the IRB’s guidelines and regulations (No. 2004-063-1116). The IRB waived the requirement for informed consent because of the retrospective nature of the study. However, written informed consent was obtained for publication of the accompanying images. The work has been reported in line with the STROCSS (Strengthening the Reporting of Cohort, Cross-sectional and Case-control Studies in Surgery) criteria [14].
We enrolled a total of 234 patients who underwent coring-out fistulectomy for cryptoglandular fistula at a tertiary hospital from January 1, 1999 to December 31, 2019, and reviewed their medical records retrospectively. The exclusion criteria were as follows: patients who were lost to follow up, unknown information about fistula type, and inflammatory bowel disease as the final pathology.
The variables assessed were demographics, fistula characteristics, intraoperative factors, and follow-up data. We classified the tracts according to Parks classification by examining the fistula tract with palpation, probe, or hydrogen peroxide (H2O2) [15].
Complex fistula was defined as follows. Tract crosses of more than 30% of the external anal sphincter (high transsphincteric with or without a high blind tract, suprasphincteric, and extrasphincteric), horse-shoe configuration, multiple tracts, recurrent, prior radiotherapy, or baseline incontinence [16]. The last follow-up day was defined as the final clinical visit. Complete healing was defined when the fistula tract was blocked and there was no discharge, and the coring-out area was epithelized.
The patient was laid in the prone position with buttock elevation. Skin preparation and draping were done (Fig. 1A). Normal saline irrigation was performed. The anal canal was sufficiently dilated to permit the introduction of a self-retaining retractor. With H2O2 injection into the fistula tract, the internal opening and tract of the fistula were identified (Fig. 1B). The incision was made around the external opening, and the tract was all cored-out along the tract from external opening to internal opening. Curettage of possibly remained inflammatory tissue was performed using a curette. Meticulous hemostasis was performed. If the excised internal opening was large, it was primarily closed with absorbable sutures. Loose seton for long-term drainage was applied along the tract after excision as per the surgeon’s decision in case of complex fistulas, or in cases of severe inflammation of surrounding tissues. Muscle filling was performed in the case of large sphincter damage. After hemostasis, marsupialization was performed with absorbable threads (Fig. 1C). Anal packing with 4 × 4 epinephrine gauze and sterile protective dressing were performed. After the operation, a stool softener and pain controller were prescribed, and patients were discharged. We followed up with patients until the wound was completely healed (Fig. 1D), questioned them on whether they had symptoms related to fecal incontinence such as gas incontinence or soiling, and recommended further visits to confirm recurrence.
The primary outcomes were the treatment success rate (recurrence rate and healing rate) and incidence of fecal incontinence. The secondary outcomes were factors affecting recurrence or nonhealing, and the differences in the recurrence and healing rates between simple and complex fistulas. Healing was defined as the complete epithelialization of the operation site without discharge. Recurrence was defined as a fistula that reoccurs around the previous surgical site after recovery.
Average and range were used to express continuous variables, and number and percentage (%) were used for expressing noncontinuous variables. To analyze factors affecting recurrence or nonhealing, patients were categorized into the primary healing group and the recurrence or nonhealing group to determine the factors affecting recurrence or healing. Univariable analysis was performed using the chi-square and Fisher exact test for categorized variables, and Student t-test and Mann-Whitney U-test for continuous variables. Logistic regression test for multivariable analysis was used when the factors were statistically significant in univariable analysis (P < 0.20). Subgroup analysis was performed for factors affecting the recurrence or nonhealing in complex type fistula in the same method as above. IBM SPSS Statistics version 25.0 for Windows (IBM Corp, Armonk, NY, USA) was used for statistical analysis. A P-value of <0.05 was considered statistically significant.
After excluding patients with inflammatory bowel disease (n = 21), those who were lost to follow up (n = 25), and those who lacked the description of tract information (n = 4), 184 patients were finally included in our study.
The baseline characteristics of the patients are described in Table 1. The average age of the patients was 44 years (range, 16–75 years), and 88.0% were male. The average body mass index was 25.3 kg/m2 (range, 16.0–38.7 kg/m2), and 24 (13.0%), 13 (7.1%), and 68 patients (37.0%) underwent operation for recurrent fistula, multiple tracts, and complex type fistula, respectively.
After coring-out fistulectomy, internal opening closure was performed by primary suture (n = 95, 51.6%) and 1 or more loose setons were placed, as appropriate, in 31 patients (16.8%). The average operation time was 22.4 minutes, and the average hospital stay was 1.2 days (range, 0–5 days).
The primary healing rate was 92.4%, and the median healing time was 58 days (interquartile range, 43–94 days). In an average of 307 days (range, 8–2,549 days) of follow-up, recurrence occurred in 15 (8.8%) out of 170 healed patients. Thus, the treatment success rate was 84.2%. There was 1 patient with fecal incontinence; however, the patient had cauda equina syndrome with fecal incontinence, which was present prior to surgery.
There was no difference in the baseline characteristics between the 2 groups (simple and complex fistula), except for smoking (19.8% vs. 36.8%, P = 0.012) (Table 1). Loose seton placement for the management of internal opening closure was performed more frequently (32.4% vs. 7.8%, P < 0.001), and the operation time was longer (29.7 minutes vs. 18.2 minutes, P < 0.001) in the complex fistula group than in the simple fistula group.
The healing rate was lower (83.8% vs. 97.4%, P = 0.001), the healing time was longer (108.1 days vs. 68.6 days, P = 0.007), and the recurrence rate was higher (22.8% vs. 1.8%, P < 0.001) among the healed patients in the complex fistula group than among those in the simple fistula group.
In the univariable analysis of the factors affecting the treatment success rate (nonhealing or recurrence), recurrent fistula (P = 0.030) and fistula types (P < 0.001) were found to be significant (Table 2). In multivariable analysis, the complex fistula (odds ratio [OR], 14.2; 95% confidence interval [CI], 4.7–43.0; P < 0.001) and internal opening closure methods (P < 0.035) were significant factors affecting treatment success (Table 2). In particular, patients in whom internal opening closure was not performed because no internal opening was detected during the operation had more recurrence and nonhealing than those who received a primary suture to the internal opening (OR, 4.0; 95% CI, 1.4–11.6; P = 0.012).
In the analysis of factors affecting the treatment success rate (nonhealing or recurrence) in complex fistula, only fistula type (P = 0.009) was a statistically significant factor (Supplementary Table 1).
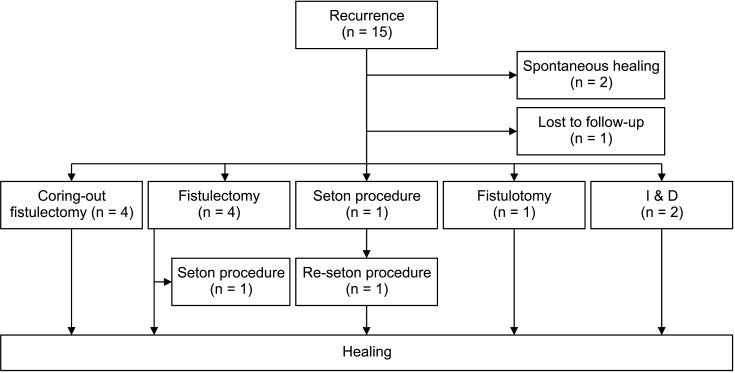
Of the 15 patients who experienced recurrence, 2 patients healed spontaneously without reoperation, and 1 patient was lost to follow up. All of the remaining 12 patients underwent reoperation. Among them, 4 patients healed after repeated coring-out fistulectomy, 3 patients healed after conventional fistulectomy. One patient healed after undergoing the seton procedure, 2 patients healed after incision and drainage, 1 patient healed after fistulotomy, and 1 patient healed after 2 seton procedures (Fig. 2).
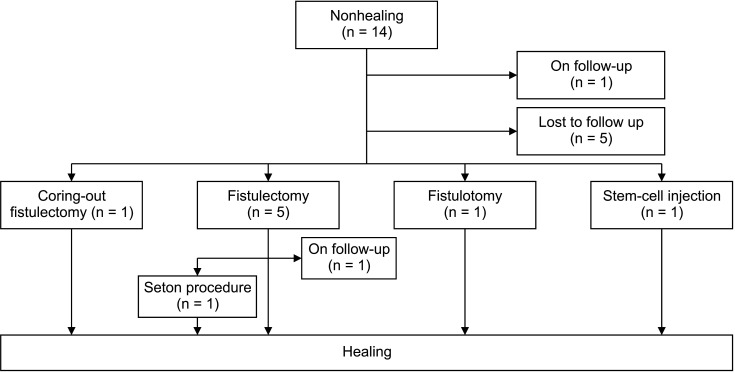
Of the 14 nonhealing patients, 1 patient was followed up and 5 patients were lost to follow up. Of the remaining 8 patients, 5 patients underwent conventional fistulectomy, 1 patient underwent coring-out fistulectomy, 1 patient underwent fistulotomy, and 1 patient underwent stem-cell injection. Of the 5 patients who underwent conventional fistulectomy, 1 patient was followed up, and 1 patient healed after the seton procedure (Fig. 3).
Among the patients who underwent reoperation due to treatment failure, coring-out fistulectomy was performed more in patients with recurrence than in nonhealing patients, but no statistical significance was found (33.3% vs. 12.5%, P = 0.603) In addition, in nonhealing patients, more conventional fistulectomy procedures were performed than in recurrence patients, but statistical significance could not be confirmed (62.5% vs. 33.3%, P = 0.362).
We demonstrated that the healing rate and recurrence rate of coring-out fistulectomy for cryptoglandular fistula were 92.4% and 8.8%, respectively, during an average of 307 days of follow-up. In particular, the healing and recurrence rates for complex cryptoglandular fistula were 83.8% and 22.8%, respectively. Moreover, when the closure was not performed because the internal opening was not detected, the recurrence and nonhealing rates were statistically significantly high with an OR of 4.00 (95% CI, 1.35–11.59).
There have been few studies on coring-out fistulectomy in the literature [1011121317]. In these studies, the healing rate was 74%–95%, and the recurrence rate was 3%–45%. Most of these studies were conducted mainly on complex fistulas, and our results were similar to those reported in these studies. However, these studies, excluding the study by Tobisch et al. [17], have a disadvantage in that the sample size was small.
So far, various sphincter-saving techniques have been published for cryptoglandular fistula. One of the commonly used techniques is LIFT [4]. Since LIFT was introduced by Rojanasakul [6] in 2009, the surgical outcomes have been reported from several studies. However, it is difficult to use it as a standard technique because, in the existing literature, this procedure was used mostly to treat transsphincteric fistulas and was rarely performed to treat intersphincteric or supra- and extrasphincteric fistulas. Moreover, there have only been 2 randomized control studies, and only the midterm success rate has been reported [7].
Most importantly, the advantage of coring-out fistulectomy is that it can accurately excise the primary fistula tract by lowering the probability of missing a secondary tract and thereby confirming the relationship between the external sphincter and track and obtaining a complete specimen [18]. However, in the case of a recurrent or severe complex fistula, after coring-out fistulectomy, simple anatomical closure may not be properly achieved due to a large defect and excessive scarring. Therefore, other sphincter-preserving methods might be additionally performed [19].
The recurrence rate of complex fistulas (22.8%) was higher than that of simple fistulas (1.8%), and the healing rate of complex fistulas was lower (83.8%) than that of simple fistulas (97.4%) after coring-out fistulectomy. In particular, among the complex types, suprasphincteric or extrasphincteric type showed a high frequency of nonhealing or recurrence. Therefore, if a fistula surgery is planned for such patients, a sufficient explanation is needed, and subsequently, attention should be paid in the follow-up period.
Fistulotomy can be easily used for a simple cryptoglandular fistula, and the frequency of recurrence after the operation has been reported to be 4%–7% [5]. Moreover, since the fecal incontinence frequency after surgery has been reported to be 0%–64% [2021], sphincter-preserving is necessary even for a simple fistula surgery. Therefore, according to the results of our study, saving the sphincter using coring-out fistulectomy can be an alternative for the treatment of simple fistula.
As reported in our study, complex fistula is a fistula type that is difficult to handle because of its higher recurrence rate and lower healing rate than those of simple fistula [2]. In complex fistulas, it is important to prevent fecal incontinence. Therefore, various techniques for this purpose have been reported. If unacceptable incontinence is expected after simple fistulotomy, a technique using advancement flap can be performed, but the success rate has been reported to be only 60% [22]. Alternatively, fibrin glue or fibrin plug is used through a fistula tract, but this cannot be applied to all complex fistulas because of the variable success rates [22]. Among the recently published surgical techniques, LIFT has commonly been used in complex fistulas. However, only 2 randomized control trials have been reported, and the majority of the literature reported only midterm success rates. Thus, its use is limited to a standard technique for complex fistula treatment [7]. Our study showed that the recurrence rate was 21.1% and the healing rate was 83.8% in the subgroup analysis of complex fistulas, which is noninferior to the 76.5% success rate reported in the meta-analysis by Emile et al. [7] as well as the results of existing literature on coring-out fistulectomy [11121317].
In our study, it was confirmed that recurrence was significantly increased in the group that did not undergo internal opening closure because no internal opening was detected. Previous studies have already reported that the recurrence rate may increase if internal openings are not found during surgery [2324]. One hypothesis is that the probability of recurrence increases because the original source of sepsis is not removed if the internal opening is not found [25]. Therefore, it is necessary to find and resolve the internal opening during anal fistula surgery.
Surgical treatment for anal fistula remains a challenge for surgeons dealing with the colorectum and anus [22]. Accordingly, various surgical techniques have been developed; however, coring-out fistulectomy as a simple and traditional method can be easily performed by anyone and can achieve similar results compared to other techniques. However, as it was difficult to detect internal openings in 31.5% of patients in our study, the detection of internal openings during surgery remains challenging. Moreover, in the case of complex fistulas, the results were similar to those of the meta-analysis of LIFT [7] and video-assisted anal fistula treatment [9]; however, the development of new surgical methods that can easily be performed by anyone and can increase the treatment success rate are necessary.
This study has some limitations. First, there was a possibility of bias because this was a retrospective study carried out at a single institution. Second, the follow-up period was relatively short (about 1 year). Nevertheless, our study is meaningful in that it summarized the experiences of a long period (about 20 years), and the analysis was conducted with a relatively large number of samples. Third, incontinence was not accurately evaluated using manometry or a certified questionnaire but was evaluated based on the patients’ self-reported symptoms. However, all patients were asked questions during the follow-up period to confirm the presence or absence of subjective symptoms. Fourth, we did not perform magnetic resonance imaging or anal endosonography. However, in most patients, the path of the fistula tract was expected as a preoperative examination. As seen in our results, it was uncommon to have a deep complex fistula tract (12.5%) such as a suprasphincteric or extrasphincteric tract. The lack of such imaging data is attributed to the cost-effectiveness of performing magnetic resonance imaging or anorectal sonography in Korea as anorectal sonography and magnetic resonance imaging were not covered by the Korean National Health Insurance Service until 2019.
In conclusion, coring-out fistulectomy is a simple and feasible technique applicable to both simple and complex fistulas. In particular, it is important to detect the internal opening and core-out to the internal opening. Furthermore, the possibility of recurrence and nonhealing after surgery for complex fistulas should be considered. However, due to the limitations of a retrospective study design conducted at a single institution, it is necessary to confirm long-term outcomes in a future multicenter study.
References
1. Zanotti C, Martinez-Puente C, Pascual I, Pascual M, Herreros D, García-Olmo D. An assessment of the incidence of fistula-in-ano in four countries of the European Union. Int J Colorectal Dis. 2007; 22:1459–1462. PMID: 17554546.

2. Wolff BC, Beck DE, Church JM, Fleshman JW, Garcia-Aguilar J, Pemberton JH. The ASCRS textbook of colon and rectal surgery. New York: Springer;2007.
3. Eisenhammer S. The internal anal sphincter and the anorectal abscess. Surg Gynecol Obstet. 1956; 103:501–506. PMID: 13360660.
4. Ratto C, Grossi U, Litta F, Di Tanna GL, Parello A, De Simone V, et al. Contemporary surgical practice in the management of anal fistula: results from an international survey. Tech Coloproctol. 2019; 23:729–741. PMID: 31368010.

5. Garcia-Aguilar J, Belmonte C, Wong WD, Goldberg SM, Madoff RD. Anal fistula surgery: factors associated with recurrence and incontinence. Dis Colon Rectum. 1996; 39:723–729. PMID: 8674361.
6. Rojanasakul A. LIFT procedure: a simplified technique for fistula-in-ano. Tech Coloproctol. 2009; 13:237–240. PMID: 19636496.

7. Emile SH, Khan SM, Adejumo A, Koroye O. Ligation of intersphincteric fistula tract (LIFT) in treatment of anal fistula: an updated systematic review, meta-analysis, and meta-regression of the predictors of failure. Surgery. 2020; 167:484–492. PMID: 31648932.

8. Meinero P, Mori L. Video-assisted anal fistula treatment (VAAFT): a novel sphincter-saving procedure for treating complex anal fistulas. Tech Coloproctol. 2011; 15:417–422. PMID: 22002535.

9. Emile SH, Elfeki H, Shalaby M, Sakr A. A systematic review and meta-analysis of the efficacy and safety of video-assisted anal fistula treatment (VAAFT). Surg Endosc. 2018; 32:2084–2093. PMID: 29052068.

11. Gustafsson UM, Graf W. Excision of anal fistula with closure of the internal opening: functional and manometric results. Dis Colon Rectum. 2002; 45:1672–1678. PMID: 12473893.
12. Jivapaisarnpong P. Core out fistulectomy, anal sphincter reconstruction and primary repair of internal opening in the treatment of complex anal fistula. J Med Assoc Thai. 2009; 92:638–642. PMID: 19459524.
13. Miller GV, Finan PJ. Flap advancement and core fistulectomy for complex rectal fistula. Br J Surg. 1998; 85:108–110. PMID: 9462397.

14. Agha R, Abdall-Razak A, Crossley E, Dowlut N, Iosifidis C, Mathew G, et al. STROCSS 2019 Guideline: strengthening the reporting of cohort studies in surgery. Int J Surg. 2019; 72:156–165. PMID: 31704426.

15. Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg. 1976; 63:1–12. PMID: 1267867.

16. Steele SR, Kumar R, Feingold DL, Rafferty JL, Buie WD. Standards Practice Task Force of the American Society of Colon and Rectal Surgeons. Practice parameters for the management of perianal abscess and fistula-in-ano. Dis Colon Rectum. 2011; 54:1465–1474. PMID: 22067173.

17. Tobisch A, Stelzner S, Hellmich G, Jackisch T, Witzigmann H. Total fistulectomy with simple closure of the internal opening in the management of complex cryptoglandular fistulas: long-term results and functional outcome. Dis Colon Rectum. 2012; 55:750–755. PMID: 22706126.

18. Weledji EP. Idiopathic anal fistula: fistulotomy or fistulectomy? Adv Res Gastroentero Hepatol. 2018; 11:555817.

19. Tozer P, Phillips RK. Anal fistula: evaluation and management. Clark S, editor. Colorectal surgery. 6th ed. Philadelphia: Elsevier;2019. p. 206–222.
20. Göttgens KW, Janssen PT, Heemskerk J, van Dielen FM, Konsten JL, Lettinga T, et al. Long-term outcome of low perianal fistulas treated by fistulotomy: a multicenter study. Int J Colorectal Dis. 2015; 30:213–219. PMID: 25421101.

21. Visscher AP, Schuur D, Roos R, Van der Mijnsbrugge GJ, Meijerink WJ, Felt-Bersma RJ. Long-term follow-up after surgery for simple and complex cryptoglandular fistulas: fecal incontinence and impact on quality of life. Dis Colon Rectum. 2015; 58:533–539. PMID: 25850841.

22. Williams G, Williams A, Tozer P, Phillips R, Ahmad A, Jayne D, et al. The treatment of anal fistula: second ACPGBI Position Statement - 2018. Colorectal Dis. 2018; 20 Suppl 3:5–31.

23. Poon CM, Ng DC, Ho-Yin MC, Li RS, Leong HT. Recurrence pattern of fistula-in-ano in a Chinese population. J Gastrointestin Liver Dis. 2008; 17:53–57. PMID: 18392245.
24. Sainio P, Husa A. Fistula-in-ano: clinical features and long-term results of surgery in 199 adults. Acta Chir Scand. 1985; 151:169–176. PMID: 4002981.
25. Abou-Zeid AA. Anal fistula: intraoperative difficulties and unexpected findings. World J Gastroenterol. 2011; 17:3272–3276. PMID: 21876613.

SUPPLEMENTARY MATERIAL
Supplementary Table 1 can be found via https://doi.org/10.4174/astr.2022.102.3.167.
Supplementary Table 1
Factors affecting nonhealing or recurrence or in complex fistulas
Fig. 1
Photographs of the anal fistula before, during, and after coring-out fistulectomy. (A) Preoperative findings. White arrow indicates external opening of the fistula. (B) Hydrogen peroxide is injected into the fistula to identify the tract and the internal opening of the fistula. (C) Marsupialization is performed with absorbable threads after coring-out fistulectomy. (D) Postoperative findings (1 month after the operation).
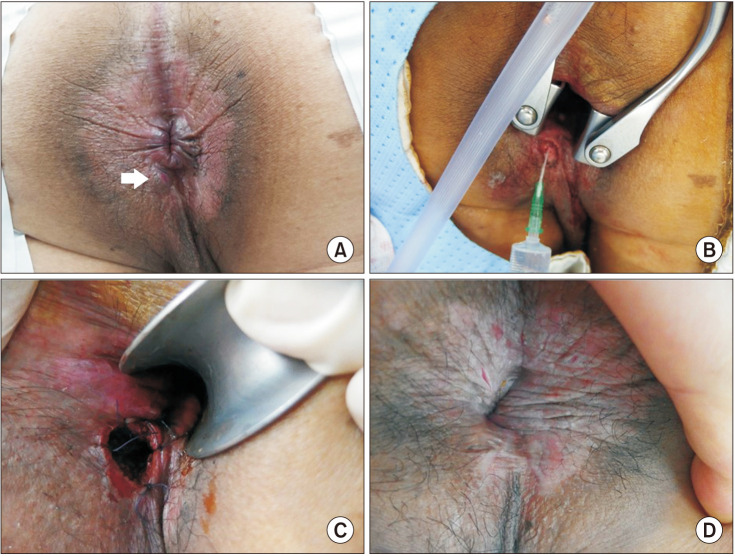
Table 1
Baseline characteristics and surgical outcomes after coring-out fistulectomy between simple and complex fistulas




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download