INTRODUCTION
With the increase of colorectal cancer incidence, surgical methods for colorectal cancer treatment are rapidly evolving. Since the introduction of minimally invasive surgical techniques, diversified laparoscopic surgical methods have been developed [
12]. Two decades ago, robotic surgery was introduced, and the speed of technological evolution has been very fast, from intraperitoneal surgery using a laparoscope to robot-assisted surgery in less than half a century [
34]. The advantages of surgical procedures using laparoscopy have been previously reported [
5]. Following this evolution, Intuitive Surgical Inc. (Sunnyvale, CA, USA) introduced a single-port robotic (SPR) device to the public in 2018. SPR is being used in various medical fields. In particular, methods for intraperitoneal surgery have been reported, and safety and feasibility have been continuously demonstrated [
67]. However, the safety and feasibility of colorectal cancer surgery using SPR have not been elucidated in detail. Moreover, it is not known how many surgeries will be required before proficiency is achieved. Notwithstanding, there is a growing interest in the recently introduced technique of SPR-assisted surgery. Although numerous studies on the learning curve of conventional robotic surgery have been published, few studies as of yet have investigated the learning curve of SPR-assisted rectal cancer surgery [
89101112].
The purpose of this study was to analyze the learning curve of SPR-assisted rectal cancer surgery using the cumulative sum (CUSUM) method, which is used as a standard indicator of skill acquisition of new technologies, and to investigate the acquisition of competency for rectal cancer operation using SPR.
Go to :

METHODS
This study was reviewed and approved by the Institutional Review Board of Samsung Medical Center (No. 2019-08-062). The study was performed in accordance with the Declaration of Helsinki and written informed consent was waived due to its retrospective nature.
From April 2019 to October 2019, 57 consecutive SPR-assisted rectal cancer surgery cases performed by one surgeon were considered in the present study. Characteristic data included the age of each patient, sex, body mass index (BMI), and American Society of Anesthesiologists (ASA) physical status (PS) classification. Intraoperative parameters included the robot setup time for each case, the time the surgeon spent using the robot, the total operation time, whether blood transfusion was performed, the estimated blood loss amount, complications during surgery, and the laparoscopic conversion rate.
Total operation time (OT) was defined as the time from skin incision at the start of surgery to skin closure at the end of surgery. Docking time (DT) was defined as the time from the end of the patient’s anesthesia induction to docking of the SPR device. Unlike laparoscopic surgery, robotic surgery requires insertion of a trocar and proper positioning of the robotic arm in the abdominal cavity through a port. Therefore, the required setup time was investigated. Cases in which the patient received total mesorectal excision (TME) were included in OT measurements. Surgeon console time (SCT) measures the time during which the surgeon executes a procedure on the console and is defined as the time spent performing surgery with the robot. The time to move and change the direction of the SPR device was included when a machine operation error occurred during robotic surgery. The surgeon had sufficient experience in single-port laparoscopic surgery but had no experience in robotic surgery. The surgical methods for rectal cancer treatment included low anterior resection (LAR), ultra-LAR, intersphincteric resection (ISR), and abdominoperineal resection (APR). In patients requiring a difficult approach to the abdomen, robot-assisted transanal TME (TaTME) was performed in parallel. All procedures were carried out with a medial to lateral approach, and high ligation was performed after confirming the origin of the inferior mesenteric artery (IMA). Dissection was performed to confer mobilization of the sigmoid colon along the avascular line in all patients. Splenic flexure mobilization was performed additionally when the sigmoid colon was short, and tension occurred in anastomosis. All patients from LAR to APR underwent TME. All anastomosis was performed intracorporeally using an EEA25-28 circular stapling device (DST SERIES EEA stapler, 3.5-mm staples; Covidien, Dublin, Ireland). If the surgical technique was changed from SPR to laparoscopy during surgery, it was classified as a laparoscopic hybrid technique. When a laparoscopic hybrid technique was performed, no additional trocars were inserted and the existing SPR port was used. Postoperative complications, including surgical site infection, anastomosis leakage, postoperative ileus, postoperative bleeding, urinary retention, complications requiring medication, and chylous drainage ascites, were measured for all cases.
Cumulative sum analysis
The learning curve was analyzed quantitatively using the CUSUM technique. CUSUM is the cumulative sum of the difference between the patient’s individual data and the mean of total cohort data [
1314]. For 7 months, OT and SCT were measured sequentially using CUSUM in 57 patients who underwent rectal cancer surgery with SPR. First, the 57 cases of SPR-assisted surgery were arranged sequentially. The CUSUM of the first data point is the difference between the first point and the average of all points, and the CUSUM after the second data point is the difference between the second point and the average of all points added to the accumulation. When the procedure time of each case is defined as Xi, the average procedure time is defined as µ, and the CUSUM of the ’N’th case is referred to as CUSUM
SPRn. The CUSUM
SPRn of each case is defined as follows.
The diagram of the CUSUM is plotted using a line chart in Microsoft Excel 2016 (Microsoft, Redmond, WA, USA). The slope of the CUSUM curve represents the trend of learning outcomes, and the regime where the slope is stabilized is regarded as the phase where the surgeon demonstrates proficiency. Additionally, the polynomial curve of CUSUM, and the values of R2 using trend line, were entered into Microsoft Excel.
Statistical analysis
In addition to CUSUM-driven analysis, the averages of the continuous variables among the patients’ characteristics were compared using 1-way analysis of variance (ANOVA). Post hoc analysis of ANOVA results was performed using the Bonferroni method. Differences in categorical variables were compared using Pearson chi-square test or Fisher exact test. Microsoft Excel 2016 was used to generate the graph of the CUSUM. For statistical processing, IBM SPSS Statistics ver. 25.0 (IBM Corp., Armonk, NY, USA) was used.
Go to :

RESULTS
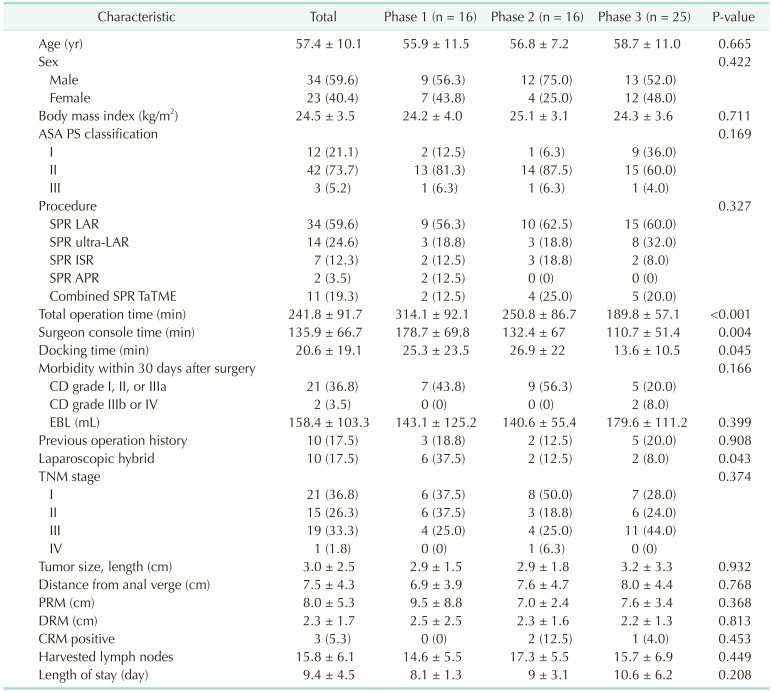
A total of 57 patients (34 males, 59.6%) were treated for rectal cancer and included 34 LAR (59.6%), 14 ultra-LAR (24.6%), 7 ISR (12.3%), and 2 APR (3.5%) cases. Among these, 11 patients additionally underwent robotic TaTME. The mean age of the patients was 57.4 ± 10.1 years, the mean BMI was 24.5 ± 3.5 kg/m2, and the median ASA PS classification was II. The mean value of total OT was 241.8 ± 91.7 minutes, the mean value of DT was 20.6 ± 19.1 minutes, and the mean value of SCT was 135.9 ± 66.7 minutes.
Total OT, SCT, and DT measured in each patient are shown in
Fig. 1. The learning curve is shown in
Fig. 2 using the CUSUM graph. The learning curve was divided into phase 1 (initial 16 cases), phase 2 (second 16 cases), and phase 3 (subsequent 25 cases). The peak of SCT in the CUSUM graph was seen in the 21st case (
Fig. 2). The longest OT among phases was in phase 2. The pathological differences between phases are shown in
Table 1. There were no statistically significant differences in age, sex, BMI, ASA PS classification, or postoperative complication among phases. However, significant differences were found in OT (P < 0.001), SCT (P = 0.004), and DT (P = 0.045). In phase 1, procedures took a longer time than those of phases 2 and 3. The average OT of procedures during phase 1 was significantly longer compared to those of phase 3 (phase 1
vs. phase 2, P = 0.070; phase 1
vs. phase 3, P < 0.001). When comparing phase 1 and phase 3 in SCT, it was found that phase 1 took significantly longer (phase 1
vs. phase 2, P = 0.113; phase 1
vs. phase 3, P = 0.003). However, when comparing with DT, there was statistically no difference between the 3 groups (phase 1
vs. phase 2, P > 0.999; phase 1
vs. phase 3, P = 0.158). There was a total of 10 surgeries with laparoscopy hybrid technique, 6 in phase 1, 2 in phase 2, and 2 in phase 3 (phase 1
vs. phase 2, P = 0.181; phase 1
vs. phase 3, P = 0.047). Additional mobilization of splenic flexure was performed in 15 of 57 (26.3%) patients. Five patients in phase 1, 4 in phase 2, and 6 in phase 3 underwent splenic flexure mobilization. Of these, 3 patients required additional laparoscopic hybrid technique. The mean time to OT was 276.33 minutes in the patient group with additional splenic flexure resection and 229.47 minutes in the group without additional splenic flexure resection.
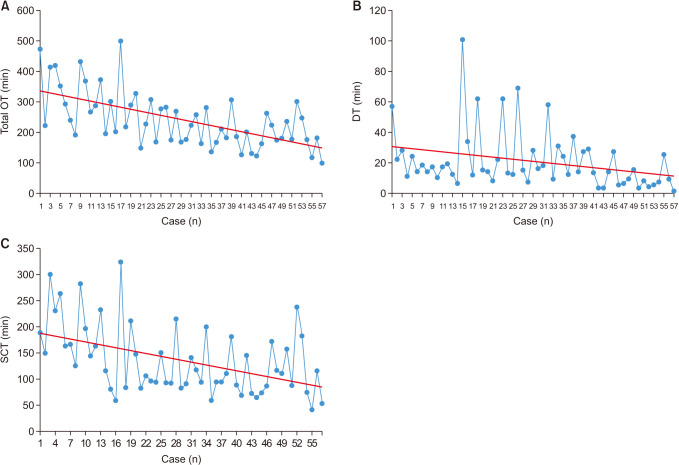 | Fig. 1(A) Operation time plotted against case number. (B) Docking time plotted against case number. (C) Surgeon console time plotted against case number. Linear trend lines are presented in red. All trend lines have negative slope. OT, total operation time; DT, docking time; SCT, surgeon console time.
|
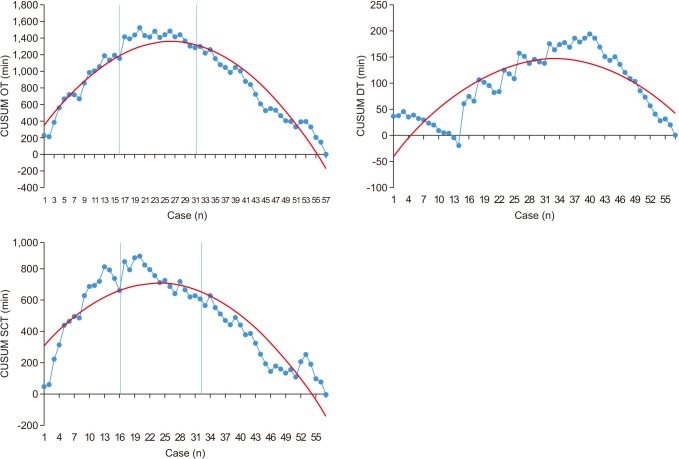 | Fig. 2Cumulative sum polynomial graph for surgeon console time and operation time of single-port robot rectal cancer surgery. On the graph, two blue lines indicate the start point of each phase. The red lines are represented by second-order polynomials, and the blue lines represent the moving average over time. OT, operation time; SCT, surgeon console time; DT, robot docking time; CUSUM, cumulative sum.
|
Table 1
Background characteristics of single-port robot colectomy (n = 57)

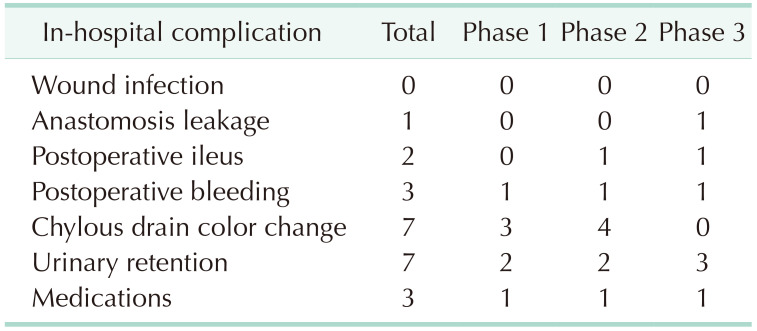
Complications were classified according to the Clavien-Dindo (CD) classification. A total of 23 complications were identified in the patients who underwent surgery and are shown in
Table 2. There were 21 patients with grade I to IIIa complications and 2 patients with IIIb to IV complications. Complications were most frequent in phase 2. However, complications of CD grade IIIb occurred most frequently in phase 3. The most common complications were fluid collection and urinary retention (7 patients each). CD grade IIIb complications comprised 1 stomal revision due to stoma obstruction and 1 irrigation and loop ileostomy due to anastomosis leakage. Due to the difficulty of the surgery, the surgical technique was changed from SPR-assisted to laparoscopic in 10 surgeries, referred to as laparoscopic hybrid surgery. Laparoscopic hybrid surgery mostly appeared in phase 1.
Table 2
Early postoperative complications of single-port robot-assisted rectal cancer surgery (n = 23)

Go to :

DISCUSSION
The methods of surgery for colorectal cancer treatment have made rapid progress in recent years. Since the advent of surgical techniques using a laparoscope, surgical methods using robots have been studied [
15]. Robotic colorectal cancer surgery has been in the spotlight in various fields of surgery as it provides convenience, 3-dimensional (3D) surgical view, and steadiness [
16]. On the flip side, laparoscopic surgical techniques continued to evolve. Single-incision laparoscopic surgery has begun to be used in various fields. In particular, many surgeries have been performed since single-incision laparoscopic surgery was proven to be safe in colorectal cancer [
17181920]. By combining these 2 technologies, a new surgical method, SPR surgery was introduced. SPR-assisted surgery has been established, making it possible to perform robotic surgery with only one incision [
212223]. Research on the learning curve of surgery using robots has been reported, though studies on the learning curve of surgery using SPR have not been published [
8910111224]. In previous studies on robotic surgery, it has been suggested that the moving average line is useful in seeing the learning curve for managing OT. In this study, we confirmed that the learning curve reached stabilization after 21 cases of surgery, representing surgical proficiency.
As in other previous studies, the learning curve for surgery was compared in detail by classifying the learning curve into 3 phases [
9]. Although the clinicopathologic characteristics among phases were not significantly different, SCT and OT significantly decreased as the surgeon’s console control skill improved through the learning phases. There was no significant difference between phase 1 and phase 2 (P = 0.070) in OT, but it was significantly reduced in phase 3 compared to phase 1 (P < 0.001). SCT was also significantly reduced (phase 1
vs. phase 2, P = 0.113; phase 1
vs. phase 3, P = 0.003). In phase 3, OT, DT, and SCT were shortened. The process of reaching SPR proficiency is thought to be due to the improvement of skill using techniques such as assessing the location of the cancer, manipulating the robot arm, controlling the 3D camera, securing the field of view, and preparing the field, all relevant to SPR. The appropriate use of additional auxiliary tools is thought to have an effect on reduction of surgery time.
Many studies divide the learning curve into 2 or 3 phases when analyzing and representing the learning curve using CUSUM. By dividing the phase into several phases, the learner can know at which point the acquisition of skills is completed. In this study, in order to find out how many surgical experiences are required until the learner can freely use the SPR device, we divided it into 3 phases. The results are also clearly shown in
Fig. 2, a graph using OT and CUSUM in
Table 1. As shown in
Fig. 2, it is interpreted that the reduction of working time and technical competence are achieved according to the learning process through the trend that peaks and decreases in the 21st case. Improvement in surgical proficiency alongside the number of surgeries, found through interpretation of the learning curve, demonstrate that experience is the most effective route to proficiency. On the other hand, it is believed that the change of docking aids played a part in the reduction of surgical duration. From the first surgery to the 12th case, GelPOINT (Applied Medical, Rancho Santa Margarita, CA, USA) was used as a docking material, and a handmade glove port was used. GelPOINT was possible to use immediately by simply assembling it when docking. However, GelPOINT was limited by the difference in the circumference or thickness of the patient’s abdomen, the change of position, the movement of the camera field of view, and the distance between the robot and the patient. Conversely, with a handmade glove port, there is a disadvantage that the robot DT increases due to the additional time required to manufacture the glove. However, even in the docking state, it was less affected by the movement of the field of view or the change in the patient’s abdominal thickness and position, helping to shorten DT. In addition, the handmade glove port was cost-effective. However, there was no statistically significant difference in DT when comparing phases 1 and 3 (phase 1
vs. phase 2, P > 0.999; phase 1
vs. phase 3, P = 0.158).
The existence of a learning curve is supported by the incidence of complications; however, there was no significant difference in complication rates between learning phases in this study. When comparing the incidence of complications by phase, complications of grade III to grade IV according to CD classification were more common in phase 3. In phase 3, ileostomy formation occurred due to stomal revision and anastomosis site leakage in patients with ileus due to stomal site malfunction. The comparison of the incidence of complications according to surgical technique should be studied further.
During SPR, when robotic access to the operation site was judged to be difficult, the OT was sufficiently longer than expected, technical difficulties occurred, and operations were performed with laparoscopic assistance. Laparoscopic surgery was performed using only a single port. In our study, the rate of performing the laparoscopic hybrid technique was 17.5% (n = 10). When comparing the 3 phases, the number of laparoscopic hybrid techniques decreased significantly when compared by phase 1 to 3 (P = 0.043). This can be explained by an increase in the proficiency in SPR-assisted surgery; compared to 6 patients treated with the hybrid technique in phase 1, 2 occurred in phase 2, and only 1 in phase 3. The entire operation was performed without a laparoscope as the skill level increased. When initially performing TME using a robot, it was difficult to approach the abdominal cavity with the robotic arm, and the field of view was determined to be limited. There was no case of open conversion during surgery.
Analysis of the CUSUM confirmed that a surgeon with experience in laparoscopic surgery using a single port needed about 21 surgical experiences to complete the learning curve of surgery using SPR. In surgeons who participated in this study, the accessibility of SPR was low considering that they had not performed previous surgery using robots. However, in learners who did not have experience in single-incision laparoscopic surgery or robot-assisted surgery, it is estimated that more cases will be needed to achieve proficiency than the number confirmed in this study. Supervision and instruction by a skilled supervisor are believed to be necessary during the journey to mastery.
The patient’s abdominal length and thickness were thought to have an effect on the DT, SCT, and OT of SPR-assisted surgery, but the relationship between these 2 factors was not confirmed as measurements according to patient shape were not obtained. These measurements are thought to be relevant for future investigations of OT and difficulty. In our experience of adjusting to the patient’s body shape during surgery, it was difficult accessing the blood vessels in cases of patients with small bodies. In particular, after the formation of pneumoperitoneum, the approach of the robot arm was not easy depending on the height from the trocar to the aorta, and ligation of the IMA was accompanied by difficulties. On the other hand, if the abdomen is too large, the distance from incision site to the rectum is increased, approach was difficult at the time of TME. Because of these differences, additional considerations are needed regarding the formation of pneumoperitoneum in appropriate patients according to body shape and size.
SPR-assisted surgery is thought to reduce pain and provide better cosmetic results as previously in laparoscopic surgery using a single port. Usually, for pain evaluation, it is possible to compare against conventional robotic operation by checking the rates of painkiller usage within 24 hours after surgery, or patient complaints using a numeric pain rating scale. However, we did not compare the pain level or pain control in patients after surgery, indicating the need for additional study.
The biggest limitation of this study is that it is difficult to generalize the results of a retrospective study conducted based on data from a single operator. In addition, it is thought that specific results were obtained in that the study specialized in SPR-assisted surgery. However, through the CUSUM, 3 phases within the learning curve, which were difficult to distinguish using the moving average line, can be distinguished relatively well. Based on this, the learning phase of unskilled surgeons can be inferred to some extent. In the future, through analysis of the learning curve using the CUSUM for several surgeons, guidelines for the learning curve of SPR-assisted rectal surgery can be determined.
In conclusion, improvement in surgical performance of SPR assisted rectal cancer operation was achieved after 21 cases. The three phases identified in the cumulative sum analysis showed a significant decrease in operative time after the middle stage of the learning curve without an increase in the complication rate.
Go to :








 PDF
PDF Citation
Citation Print
Print



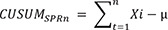

 XML Download
XML Download