Abstract
Purpose
Pancreatic enzyme reflux into the biliary tract is associated with chronic inflammation and increased cellular proliferation in the biliary epithelium, leading to biliary carcinoma. We evaluated the relationship between high bile juice amylase levels and biliary microflora in patients with malignant gallbladder lesions.
Methods
In this retrospective study, 25 gallbladder specimens were obtained from patients with gallbladder cancer to evaluate amylase levels and perform bacterial culture. The samples were divided into high and low amylase groups and culture-positive and negative groups for analysis. Bile juice amylase 3 times higher than the normal serum amylase level (36–128 IU/L) was considered high.
Results
The number of positive cultures was higher in the high amylase group than in the low amylase group, but the difference was insignificant. There were no differences in other clinicopathological factors. Sixteen patients showed positive culture results; Escherichia coli and Klebsiella spp. were the most common gram-negative bacteria, whereas Enterococcus and Streptococcus spp. were the most common gram-positive bacteria. Age and bile juice amylase levels were significantly higher in the culture-positive group than in the culture-negative group. The incidence of bacterial resistance to cephalosporins was 6.25%–35.29%, and this incidence was particularly high for lower-generation cephalosporins.
Go to : 
Pancreatic juice reflux into the biliary tract usually occurs in patients with an anomalous pancreaticobiliary ductal union (APBDU), and a mixture of pancreatic and bile juices in the gallbladder is associated with a high incidence of gallbladder cancer (GBC) [1]. Occult pancreaticobiliary reflux (OPBR) is characterized by high levels of biliary amylase in a morphologically normal pancreaticobiliary duct due to pressure differences between the sphincter of Oddi and the duodenum. A spastic sphincter of Oddi and the lack of sphincter function are associated with pancreatic juice reflux [23]. In our previous study, we found that a high amylase level in the gallbladder was a risk factor for inflammation and degeneration of the gallbladder mucosa [4]. Pancreatic enzyme reflux into the biliary tract is also associated with chronic inflammation and increased cellular proliferation of the biliary epithelium, leading to biliary carcinoma. Chronic bacterial infections in the bile leading to the production of carcinogenic precursors may be an etiologic factor in GBC pathogenesis [5678]. However, little research has been conducted on clinicopathological factors in patients with OPBR and bacterial infections. Therefore, our study aimed to determine the prevalence of pancreatic juice reflux in patients with GBC and its association with chronic bacterial infections.
Go to : 
This study was approved by the Institutional Review Board of Pusan National University Hospital (No. 2110-005-107) and followed the guidelines of the Declaration of Helsinki. All patients provided written informed consent prior to participation. All patients also provided written consent for the publication of their data.
From 2013 to 2019, 117 patients with GBC (including carcinoma in situ) who underwent elective laparoscopic cholecystectomy or radical cholecystectomy at Pusan National University Hospital were identified. Patients who underwent preoperative endoscopic retrograde cholangiography or percutaneous transhepatic gallbladder drainage, patients who showed bile duct dilatation of >1.0 cm, or patients with choledochal cysts or other malignancies were excluded. Bile samples were obtained from the resected gallbladder specimens, and amylase and lipase levels were measured. Patients were categorized into high and low amylase groups. Bacterial cultures were also performed, and patients were divided into culture-positive and culture-negative groups. The clinicopathological factors of patients were evaluated. During follow-up, one patient died of cancer recurrence.
Immediately after cholecystectomy, bile and blood samples were collected. A gallbladder amylase level more than 3 times higher than the serum amylase level (normal range, 36–128 IU/L) was considered high. Bile juice amylase levels could be measured in the range of 3–5,250 IU/L. Intraoperative biliary samples were collected under aseptic conditions at the time of bacteriological examinations during the operation. The cultures were evaluated for prevalence of aerobic, anaerobic, and fungal organisms using routine biochemical tests. Different antimicrobial panels were used depending on the isolated organism, and the isolates were classified as susceptible, intermediate, or resistant, according to the Clinical and Laboratory Standards Institute document M47-A (2007; https://clsi.org/media/1448/m47a_sample.pdf) principles and procedures for blood cultures.
All data are presented as mean values. The groups (low vs. high amylase and culture-positive vs. culture-negative) were compared using the Mann-Whitney U-test, and categorical data were compared between groups using the chi-square test. Statistical significance was defined as a P-value of <0.05. IBM SPSS Statistics ver. 23 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses.
Go to : 
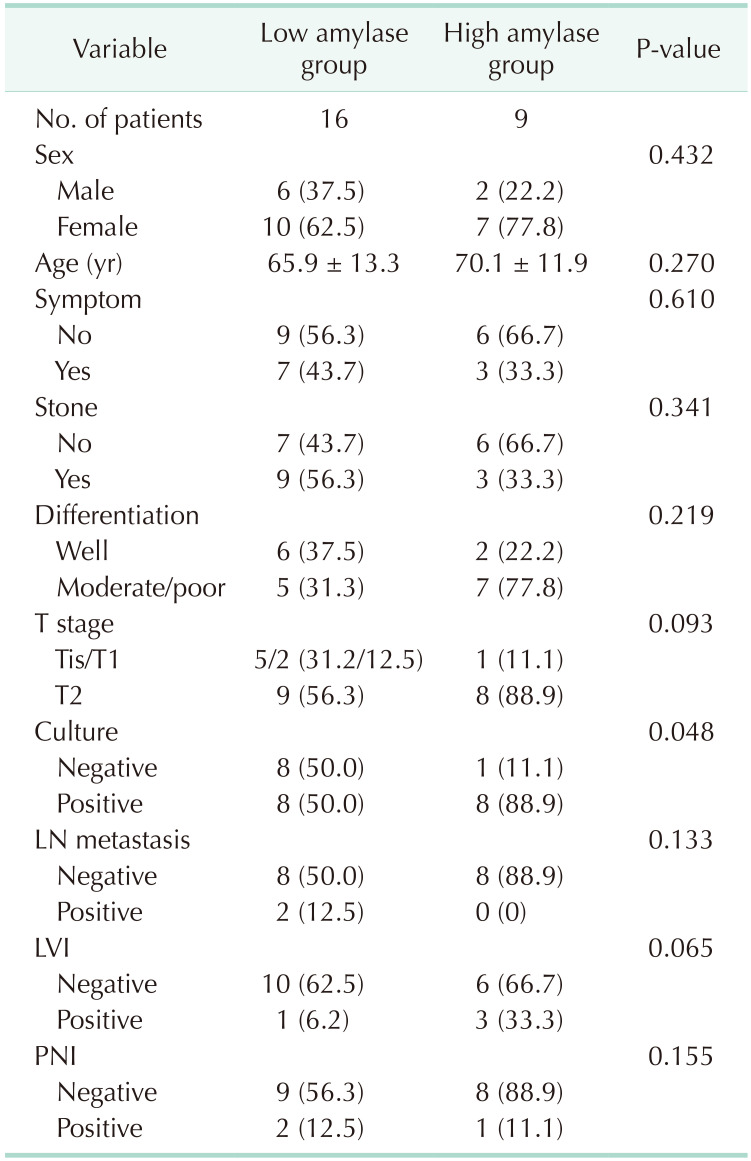
A total of 25 patients were enrolled in this study. The mean patient age was 65.9 years in the low amylase group and 70.1 years in the high amylase group. Overall, 12 patients had gallbladder stones. Abdominal pain was the most common symptom (10 patients), but it was not a symptom of acute cholecystitis. There were no patients with acute cholecystitis. A high amylase level was more frequent in GBCs with tumor stage of >T2, but the difference between the high and low amylase groups was not significant (88.9% vs. 56.2%, P = 0.093). The high amylase group had a higher number of positive cultures than the low amylase group (88.9% vs. 50.0%, P = 0.052), but this difference was also insignificant. There were no significant differences in other clinicopathological factors (Table 1).
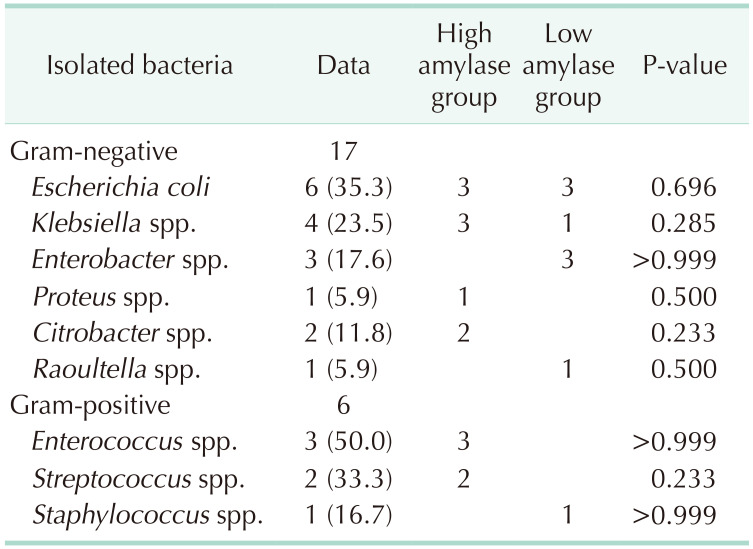
Sixteen patients showed positive culture results (64.0%). Escherichia coli and Klebsiella spp. were the most common gram-negative bacteria, whereas Enterococcus and Streptococcus spp. were the most common gram-positive bacteria. Two or more strains were detected in 4 patients, all of whom were from the high amylase group, and 3 of these 4 patients had both gram-positive and gram-negative bacteria. There was no difference in the frequency between the 2 groups (Table 2).
When differences in clinicopathological factors between the culture-negative and culture-positive groups were analyzed, age (72.6 years vs. 58.2 years, P = 0.005) and the proportion of patients with high amylase levels (50.0% vs. 11.1%, P = 0.048) were higher in the culture-positive group than in the culture-negative group. But there were no significant differences in the other clinicopathological factors (Table 3).
E. coli and Klebsiella spp. were the most common gram-negative bacteria, whereas Enterococcus and Streptococcus spp. were the most common gram-positive bacteria. For ampicillin, penicillin, and first-generation cephalosporin families, the incidence of resistance was greater than 30%. The incidence of resistance to the cephalosporin series of antibiotics ranged between 6.25% and 35.29%. They had a high rate of antimicrobial resistance to first- or second-generation cephalosporins. Amikacin, ertapenem, imipenem, and trimethoprim/sulfamethoxazole are the most suitable antibiotics.
Go to : 
Cellular proliferation of the biliary epithelium is facilitated by the regurgitation of pancreatic juice into the bile duct in patients with APBDU, and it caused genetic alterations in the bile duct. Repetitive regurgitation of pancreatic juice into the bile duct can induce a hyperplastic or dysplastic state in the biliary tract, potentially leading to cancerous changes in the mucosa [910]. Previous studies have indicated that changes in the biliary mucosa also occur in patients with an anatomically normal pancreaticobiliary junction; this condition is called OPBR [111213141516]. High biliary amylase levels can be observed in patients with OPBR; these are sometimes associated with biliary malignancies, similar to that noted in patients with APBDU [111718].
With respect to APBDU and OPBR, there is a limitation regarding preoperative diagnosis; therefore, researchers are less interested in such studies. According to Itokawa et al. [17], the bile juice amylase level in the gallbladder is equal to or higher than that in the common bile duct. Thus, the amylase level in the gallbladder may indicate OPBR, which would imply that the mechanism underlying APBDU also causes GBC.
However, the definition of OPBR is potentially controversial, and no cutoff values have been established. Itokawa et al. [17] reported an OPBR frequency of 26% in patients with various pancreaticobiliary diseases, and the incidence of OPBR in patients with GBC was 36.0% (9 of 25), according to their OPBR definition. Beltrán et al. [18] defined OPBR as a higher amylase level in the gallbladder than that in the serum and reported the incidence of OPBR as 84.2% in patients with benign gallbladder diseases and 100% in patients with GBC. In our study, OPBR was defined as a gallbladder amylase level more than 3 times higher than the serum amylase level, based on the definition for postoperative pancreatic fistulas.
The incidence of APBDU in patients with biliary disorders who underwent endoscopic retrograde cholangiography is 1.5% in Japan, 8.7% in Taiwan, and 1% in Europe. In the last decade, the incidence of APBDU-associated bile duct cancer in patients with and without biliary dilatation was 5.2% and 4.0%, respectively. Several studies have reported that biliary cancer is detected in 21.6% of adult patients with congenital biliary dilatation (bile duct cancer, 32.1% vs. GBC, 62.3%) and in 42.4% of patients with pancreaticobiliary maljunction without biliary dilatation (bile duct cancer, 7.3% vs. GBC, 88.1%) [19202122]. In our previous study, we recommended that patients with high bile amylase levels after cholecystectomy should be carefully followed up for cholangiocarcinoma [4]. However, there were no remarkable changes in these groups during the follow-up period. We speculated that this was because of the low incidence of bile duct cancer among patients with APBDU without bile duct dilatation. Additionally, the risk of bile duct cancer decreased after cholecystectomy; similar to a decrease in the risk of intrapancreatic bile duct cancer after choledochal cyst operation. However, the incidence of GBC in patients with APBDU without bile duct dilatation was high (88.1%) in our study, which could not be explained by pancreatic juice reflux alone. And we could not evaluate the presence of APBDU separately, so it was necessary to identify other clinicopathological factors related to GBC in patients with APBDU or OPBR without bile duct dilatation.
If OPBR presents with a functional problem related to the sphincter of Oddi, it may cause chronic bacterial infections owing to bile contamination caused by reflux from the duodenum into the biliary tract. Therefore, chronic bacterial infection in the bile has been suggested as a trigger for GBC. Several studies have reported that infection caused by specific organisms, such as Salmonella typhi, is a leading cause of GBC [5678]. The reported bacterial infection rate is 9%–42% during elective cholecystectomy, 35%–65% in the presence of symptomatic acute cholecystitis, and 30%–90% during biliary drainage treatment [23]. In our study, the incidence of bacterial infection was 64%, which is higher than the 25.1% incidence during elective cholecystectomy for benign gallbladder disease reported in our previous study. In our present study, in the low amylase group, the positive culture rate was 50%. We could not identify exactly the reason for the positive culture rate in the low amylase group, but we can assume the possibilities of contamination. The patients with GB stone in the low amylase group were 56.3%. So we could guess the chronic inflammation with bacterial infection in these patients even if they had not OPBR. Bacterial infections were also more frequent in the high amylase group than in the low amylase group, indicating an association between OPBR and chronic bacterial infections (P = 0.048). Considering that OPBR can be a risk factor for GBC, we suggest a correlation between GBC and chronic bacterial infection in the gallbladder. Further, because the incidence of bile culture positivity was higher in patients with a high amylase level and in older patients, we hypothesize that alterations in the gallbladder mucosa due to chronic bacterial infections in patients with OPBR increase the risk of cancer.
In our study, the most commonly identified bacteria were E. coli, Klebsiella spp., and Enterococcus spp. (same as those detected during elective cholecystectomy). The frequency of gram-positive bacteria was higher in the high amylase group than in the low amylase group, but the difference was not significant. The role of prophylactic antibiotics in cancer surgery remains controversial. Antibiotic susceptibility studies have suggested that amikacin, ertapenem, imipenem, and trimethoprim/sulfamethoxazole are the most suitable antibiotics, but their use is limited in clinical applications. When considering antibiotic administration in the perioperative period, first- or second-generation cephalosporins should be avoided owing to their high rate of antimicrobial resistance.
This study has some limitations. The objective was to demonstrate the relationship between high bile amylase levels and chronic bacterial infections in patients with GBC. The study had a retrospective design and included only 25 patients with GBC. The degree of inflammation surrounding the cancer tissue was not evaluated, and the groups were not balanced according to the cancer stage. Furthermore, we could not determine the appropriate antibiotic treatment for patients because the bacterial status in the bile duct after surgery was not evaluated. The antimicrobial resistance of the isolated bacteria depends on several factors, including community differences and hospital antibiotic policies, and consequently, the antibiotic management should be adjusted considering these environmental factors.
However, chronic bacterial infections associated with OPBR may be correlated with GBC, and future studies, including biochemical and bacterial gallbladder bile fraction evaluation, are warranted for confirmation.
In patients with GBC, the clinicopathological factors did not differ according to the bile juice amylase levels. However, bacteria in gallbladder were identified more frequently when the amylase level was high. High amylase levels in the gallbladder can be associated with caused chronic bacterial infections with OPBR, potentially triggering GBC.
Go to : 
References
1. Hanada K, Itoh M, Fujii K, Tsuchida A, Hirata M, Ishimaru S, et al. Pathology and cellular kinetics of gallbladder with an anomalous junction of the pancreaticobiliary duct. Am J Gastroenterol. 1996; 91:1007–1011. PMID: 8633539.
2. Schweizer P, Schweizer M. Pancreaticobiliary long common channel syndrome and congenital anomalous dilatation of the choledochal duct: study of 46 patients. Eur J Pediatr Surg. 1993; 3:15–21. PMID: 8466869.

3. Imazu M, Iwai N, Tokiwa K, Shimotake T, Kimura O, Ono S. Factors of biliary carcinogenesis in choledochal cysts. Eur J Pediatr Surg. 2001; 11:24–27. PMID: 11370978.

4. Yun SP, Lee JY, Jo HJ, Kim HS, Kim DH, Kim JH, et al. Long-term follow-up may be needed for pancreaticobiliary reflux in healthy adults. J Korean Surg Soc. 2013; 84:101–106. PMID: 23397015.

5. Sharma V, Chauhan VS, Nath G, Kumar A, Shukla VK. Role of bile bacteria in gallbladder carcinoma. Hepatogastroenterology. 2007; 54:1622–1625. PMID: 18019679.
6. Goetze TO. Gallbladder carcinoma: prognostic factors and therapeutic options. World J Gastroenterol. 2015; 21:12211–12217. PMID: 26604631.

7. Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014; 6:99–109. PMID: 24634588.
8. Espinoza JA, Bizama C, García P, Ferreccio C, Javle M, Miquel JF, et al. The inflammatory inception of gallbladder cancer. Biochim Biophys Acta. 2016; 1865:245–254. PMID: 26980625.

9. Funabiki T, Matsubara T, Miyakawa S, Ishihara S. Pancreaticobiliary maljunction and carcinogenesis to biliary and pancreatic malignancy. Langenbecks Arch Surg. 2009; 394:159–169. PMID: 18500533.

10. Tanno S, Obara T, Fujii T, Mizukami Y, Shudo R, Nishino N, et al. Proliferative potential and K-ras mutation in epithelial hyperplasia of the gallbladder in patients with anomalous pancreaticobiliary ductal union. Cancer. 1998; 83:267–275. PMID: 9669809.

11. Sai JK, Suyama M, Kubokawa Y, Tadokoro H, Sato N, Maehara T, et al. Occult pancreatobiliary reflux in patients with a normal pancreaticobiliary junction. Gastrointest Endosc. 2003; 57:364–368. PMID: 12612517.

12. Sai JK, Suyama M, Nobukawa B, Kubokawa Y, Sato N. Severe dysplasia of the gallbladder associated with occult pancreatobiliary reflux. J Gastroenterol. 2005; 40:756–760. PMID: 16082594.

13. Vracko J, Markovic S, Wiechel KL. Conservative treatment versus endoscopic sphincterotomy in the initial management of acute cholecystitis in elderly patients at high surgical risk. Endoscopy. 2006; 38:773–778. PMID: 17001566.

14. Sai JK, Suyama M, Nobukawa B, Kubokawa Y, Yokomizo K, Sato N. Precancerous mucosal changes in the gallbladder of patients with occult pancreatobiliary reflux. Gastrointest Endosc. 2005; 61:264–268. PMID: 15729237.

15. Vracko J, Wiechel KL. Increased gallbladder trypsin in acute cholecystitis indicates functional disorder in the sphincter of oddi and could make EPT a logical procedure. Surg Laparosc Endosc Percutan Tech. 2003; 13:308–313. PMID: 14571164.

16. Vracko J, Zemva Z, Pegan V, Wiechel KL. Sphincter of Oddi function studied by radioimmunoassay of biliary trypsin in patients with bile duct stones and in controls. Surg Endosc. 1994; 8:389–392. PMID: 8073354.

17. Itokawa F, Itoi T, Nakamura K, Sofuni A, Kakimi K, Moriyasu F, et al. Assessment of occult pancreatobiliary ref lux in patients with pancreaticobiliary disease by ERCP. J Gastroenterol. 2004; 39:988–994. PMID: 15549453.

18. Beltrán MA, Vracko J, Cumsille MA, Cruces KS, Almonacid J, Danilova T. Occult pancreaticobiliary reflux in gallbladder cancer and benign gallbladder diseases. J Surg Oncol. 2007; 96:26–31. PMID: 17345616.

19. Matsuda T, Marugame T, Kamo K, Katanoda K, Ajiki W, Sobue T, et al. Cancer incidence and incidence rates in Japan in 2003: based on data from 13 population-based cancer registries in the Monitoring of Cancer Incidence in Japan (MCIJ) Project. Jpn J Clin Oncol. 2009; 39:850–858. PMID: 19797417.

20. Kamisawa T, Kuruma S, Tabata T, Chiba K, Iwasaki S, Koizumi S, et al. Pancreaticobiliary maljunction and biliary cancer. J Gastroenterol. 2015; 50:273–279. PMID: 25404143.

21. Sastry AV, Abbadessa B, Wayne MG, Steele JG, Cooperman AM. What is the incidence of biliary carcinoma in choledochal cysts, when do they develop, and how should it affect management? World J Surg. 2015; 39:487–492. PMID: 25322698.

22. Matsuda M, Watanabe G, Hashimoto M, et al. Evaluation of pancreaticobiliary maljunction and low bile amylase. J Jpn Biliary Assoc. 2007; 21:119–124.
23. Darkahi B, Sandblom G, Liljeholm H, Videhult P, Melhus Å, Rasmussen IC. Biliary microflora in patients undergoing cholecystectomy. Surg Infect (Larchmt). 2014; 15:262–265. PMID: 24801654.

Go to : 




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download