Abstract
Ameloblastoma is a histologically benign odontogenic epithelial tumor that rarely metastasizes. However, metastasis to the lungs can occur, usually years after the development of the primary tumor. Here, we present the case of a 63-year-old woman with metastatic ameloblastoma in the lungs that developed 12 years after surgery for the primary lesion. As is typical for metastatic ameloblastomas, the tumor was incidentally found on radiography and surgically removed. However, the tumor exhibited accelerated progression with pleural metastasis after surgical removal, which is unusual in metastatic ameloblastoma. The patient was successfully treated with carboplatin/paclitaxel and showed a partial response to tumor progression, implying that this approach can be safely used in the absence of a standard treatment regimen.
Ameloblastoma is the second most common odontogenic tumor that mainly involves the mandible and maxilla [1]. It is a benign tumor arising from the odontogenic epithelium [2], with slow invasive growth and a high recurrence rate (50%–72%). Although rare (<2%), metastasis is primarily distant [1]. Histopathologically, metastatic ameloblastoma does not differ from non-metastatic ameloblastoma, making it impossible to predict its clinical behavior [3]. The most common site of metastasis is the lungs (≥5%) [4]. The current treatment choice for primary ameloblastoma is surgery, including both curettage and radical surgery. However, the treatment for metastatic ameloblastoma is yet to be established [3,4].
We present a case of metastatic ameloblastoma which developed in the patient’s lung 12 years after a primary lesion surgery. This case is unusual owing to the rapid tumor progression with pleural metastasis that was observed after the resection of metastatic ameloblastoma of the lung. Due to the absence of a regular treatment regimen, the patient was treated with chemotherapy using carboplatin and paclitaxel.
Ethical statements: This study was approved by the Institutional Ethics Committee of Wonkwang University Hospital (WKUH 2020-06-015-001). Informed consent was obtained from the patient.
In March 2020, a 63-year-old woman with pulmonary nodules was referred to the Wonkwang University Hospital, Iksan, Korea. She had a history of recurrent ameloblastoma of the right mandibular ramus, for which she underwent mass excision and bone grafting in 1983 and 2001. The pulmonary nodules were incidentally found on a regular chest radiography in 2013. A chest computed tomography (CT) scan revealed two lesions in the right lung, a 13 mm-sized heterogeneous enhancing solid nodule with peripheral ground-glass opacity (GGO) in the right lower lobe (RLL) (Fig. 1A), and an 8 mm-sized noncalcified nodule in the right middle lobe (RML) (Fig. 1B). The patient refused the recommended diagnosis and treatment. She returned asymptomatic in March 2020 with enlarged pulmonary nodules and agreed to undergo treatment.
The patient had a normal physical examination. On the CT scan, both the nodules had remarkably increased in size. The RLL mass was 45 mm in size, well-defined, lobulated, and heterogeneously enhanced with peripheral GGO; it abutted the right major fissure and diaphragm, causing pleural retraction (Fig. 2A). The RML mass was 14 mm in size, spiculated-margined, and solid enhancing, which also caused pleural retraction (Fig. 2B). Considering her past diagnosis, metastatic ameloblastoma was more likely than a primary lung cancer with a metastatic nodule. We performed resection of the anterior basal segment of the RLL and wedge resection of the RML. Postoperative histopathological evaluation confirmed the diagnosis (Fig. 3). No histological signs of malignancy were observed. Positron emission tomography with CT only showed signs of postoperative pulmonary inflammation. The patient was discharged without indication for adjuvant therapy.
In July 2020 she was admitted to the emergency room with mild dyspnea (mMRC 1 grade). Chest X-ray revealed a massive right pleural effusion. Analysis of the aspirated pleural fluid showed a lymph-dominant exudate and suspected malignant pleural effusion, but the cytological examination was negative for malignancy. After drainage of the pleural effusion, a chest CT was performed, which showed locoregional tumor recurrence at the surgical stump site with multiple pleural metastases in the right hemithorax (Fig. 4A). Subsequently, thoracoscopic pleural biopsy was performed. Pathology confirmed metastatic ameloblastoma. Chemotherapy is recommended for multiple pleural metastases and lesions that are not amenable to surgical resection or radiotherapy. Our multidisciplinary tumor board decided to treat with combined chemotherapy of carboplatin and paclitaxel for six cycles which were administered intravenously every 21 days. After three cycles, CT scans were taken, which showed variable responses to the chemotherapy. Three additional cycles were administered. Patient developed grade 4 neutropenia during the last cycle but recovered. Post-treatment, follow-up CT scans demonstrated a radiological partial response (Fig 4B). A year later, the patient is clinically asymptomatic.
Among odontogenic and maxillofacial bone tumors, ameloblastomas (11%) are the second most common neoplasm [5]. Despite its local invasiveness and frequent recurrence, ameloblastoma is benign, slow-growing, and asymptomatic. Primary ameloblastomas are usually diagnosed between the third and fifth decades of life without any race or gender predilections [3,6].
Despite its 50% to 72% rate of relapse, metastasis to distant organs or lymph nodes rarely occur [7]. Distinguishing metastatic ameloblastoma from ameloblastic carcinoma is important. In 2017, the World Health Organization classified metastatic ameloblastoma as a benign odontogenic tumor [8]. Primary and metastatic ameloblastomas are histopathologically identical to benign ameloblastomas. The histologic findings observed in the primary tumor are not specific for predicting metastasis [8]. The known risk factors of metastasis are the tumor duration, the extent of initial disease, multiple surgical procedures, and radiation therapy [4,9].
The average disease-free interval is 18 years (range, 3–45 years) [6]. In this case, it was 12 years. Biopsy is needed to confirm the diagnosis of malignant ameloblastoma. As in our case, it is often found incidentally by imaging studies in asymptomatic patients. Regular radiographic follow-ups of asymptomatic patients with a history of ameloblastoma are important for early detection.
When our patient returned in 2020, we observed that the metastatic ameloblastoma had grown relatively slow since 2013. Three months after the surgical removal, unusual rapid tumor progression with pleural metastasis was observed in the metastatic ameloblastoma. Even considering the possibility of recurrence at the surgical stump, this is the first reported case of accelerated postoperative growth in metastatic ameloblastoma. There is a risk of accelerated growth of metastatic disease and increased formation of new metastatic foci in the perioperative period. However, the underlying mechanism remains unclear. There are a few hypotheses, such as surgery inducing the upregulation of adhesion molecules in target organs and recruiting immune cells capable of entrapping tumor cells [10]. Furthermore, surgery can induce changes in the target tissue and in the cancer cells to enhance migration and invasion to the target site [10]. Surgical trauma can induce local and systemic inflammatory responses, which can contribute to the accelerated growth of residual and metastatic disease [11].
Treatment of choice for primary ameloblastoma is surgical resection, while treatment for metastatic ameloblastoma remains controversial [3,12]. The evidence for treatment decisions has mostly been derived from case reports due to the disease rarity [1]. Surgical resection is the treatment for pulmonary metastasis. Chemotherapy or radiotherapy have been indicated for non-resectable lesions, but the effects are not well-studied [9,12]. Since the effects of chemotherapy are unpredictable, no single-agent or combination regimen has been recommended [1]. Radiotherapy is only for palliative care [4]. Amzerin et al. [5] reviewed the responses of chemotherapy in less than 50 cases. They recommended platinum-based regimens as first-line treatment. Therefore, we treated our patient with carboplatin and paclitaxel. She achieved partial response after six cycles. Fortunately, metastatic ameloblastoma of the lung and pleura was reduced by over 90%.
Metastatic ameloblastoma is typically asymptomatic and discovered incidentally, as in this case. The highlight of our case was the postoperative accelerated tumor growth of metastatic ameloblastoma after metastasectomy. When surgical resection was impossible, we treated with well-tolerated carboplatin and paclitaxel. In the absence of established treatment for metastatic ameloblastoma, chemotherapy using carboplatin and paclitaxel can be considered.
Notes
Author contributions
Conceptualization: JWJ. Data curation: JUP, JWJ. Formal analysis: JUP, JWJ. Funding acquisition: JWJ. Methodology: JUP. Project administration: JWJ. Visualization: JUP. Writing - original draft: JUP. Writing - review & editing: JUP, JWJ. Approval of final manuscript: all authors.
References
1. Ghiam A, Al Zahrani A, Feld R. A case of recurrent metastatic ameloblastoma and hypercalcaemia successfully treated with carboplatin and paclitaxel: long survival and prolonged stable disease. Ecancermedicalscience. 2013; 7:323.
2. Li D, Xu S, Sun M, Qiao L, Wang L, Liu Y. MAID chemotherapy regimen as a treatment strategy for metastatic malignant ameloblastoma: a case report. Medicine (Baltimore). 2019; 98:e15873.
3. Mendenhall WM, Werning JW, Fernandes R, Malyapa RS, Mendenhall NP. Ameloblastoma. Am J Clin Oncol. 2007; 30:645–8.

4. Valkadinov I, Conev N, Dzhenkov D, Donev I. Rare case of ameloblastoma with pulmonary metastases. Intractable Rare Dis Res. 2017; 6:211–4.

5. Amzerin M, Fadoukhair Z, Belbaraka R, Iraqui M, Boutayeb S, M'rabti H, et al. Metastatic ameloblastoma responding to combination chemotherapy: case report and review of the literature. J Med Case Rep. 2011; 5:491.

6. Van Dam SD, Unni KK, Keller EE. Metastasizing (malignant) ameloblastoma: review of a unique histopathologic entity and report of Mayo Clinic experience. J Oral Maxillofac Surg. 2010; 68:2962–74.

7. Inoue N, Shimojyo M, Iwai H, Ohtsuki H, Yasumizu R, Shintaku M, et al. Malignant ameloblastoma with pulmonary metastasis and hypercalcemia. Report of an autopsy case and review of the literature. Am J Clin Pathol. 1988; 90:474–81.

8. Wright JM, Vered M. Update from the 4th edition of the World Health Organization Classification of Head and Neck Tumours: odontogenic and maxillofacial bone tumors. Head Neck Pathol. 2017; 11:68–77.

9. Senra GS, Pereira AC, Murilo dos Santos L, Carvalho YR, Brandao AA. Malignant ameloblastoma metastasis to the lung: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008; 105:e42–6.

10. Tohme S, Simmons RL, Tsung A. Surgery for cancer: a trigger for metastases. Cancer Res. 2017; 77:1548–52.

Fig. 1.
Initial chest computed tomography performed in October 2013. (A) A 13-mm heterogeneous enhancing solid nodule with a peripheral ground-glass opacity in the right lower lobe (arrow). (B) An 8-mm small, noncalcified nodule in the right middle lobe (arrow).
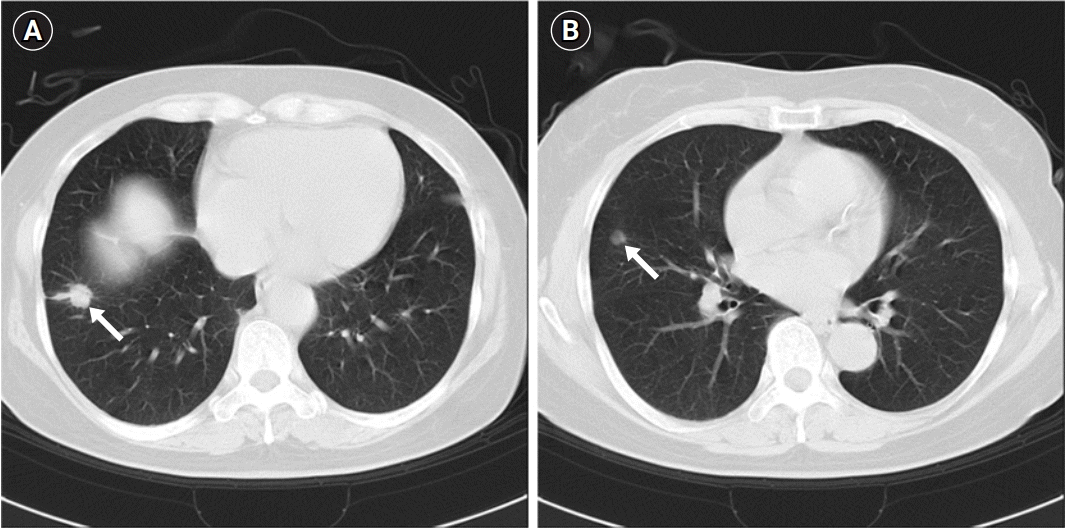
Fig. 2.
Follow-up chest computed tomography performed in March 2020. (A) A 45-mm well-defined, lobulated, heterogeneously enhancing mass with a peripheral ground-glass opacity, abutting the right major fissure and diaphragm and causing pleural retraction in the right lower lobe (arrow). (B) A 14-mm, spiculated-margin, enhancing solid nodule with pleural retraction in the right middle lobe (arrow).
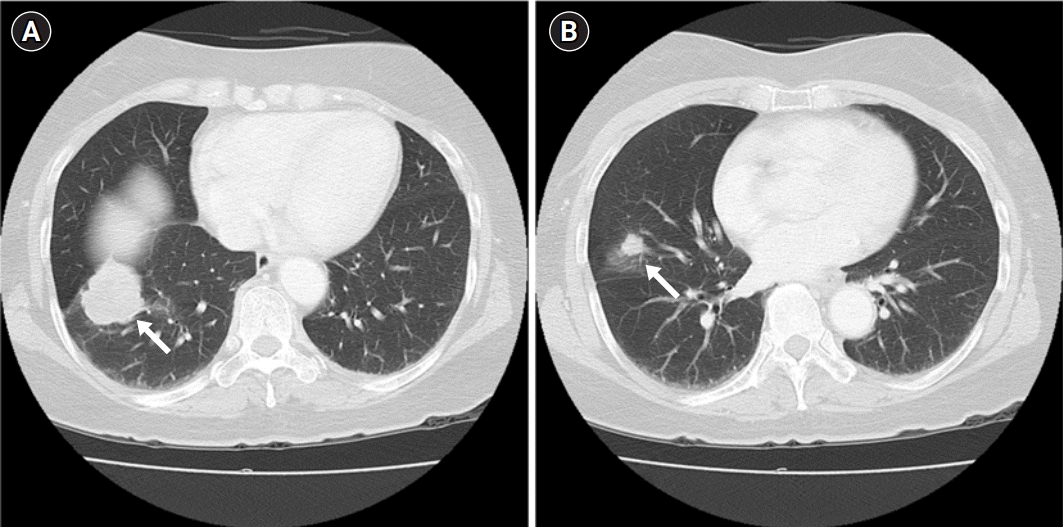
Fig. 3.
Pathological findings of the lung biopsy specimen. (A) The pulmonary metastatic lesion is more cellular, with a clear margin between the lesion and the surrounding lung tissue (H&E, ×40). (B) Anastomosing epithelial strands show peripheral palisading (arrows) with more loosely arranged angular cells (arrowheads) and variable keratinization (asterisk) (H&E, ×100). (C) CD56 is preferentially expressed in peripheral cells, and (D) CK19 is expressed in all cells (immunohistochemical stain, ×100).
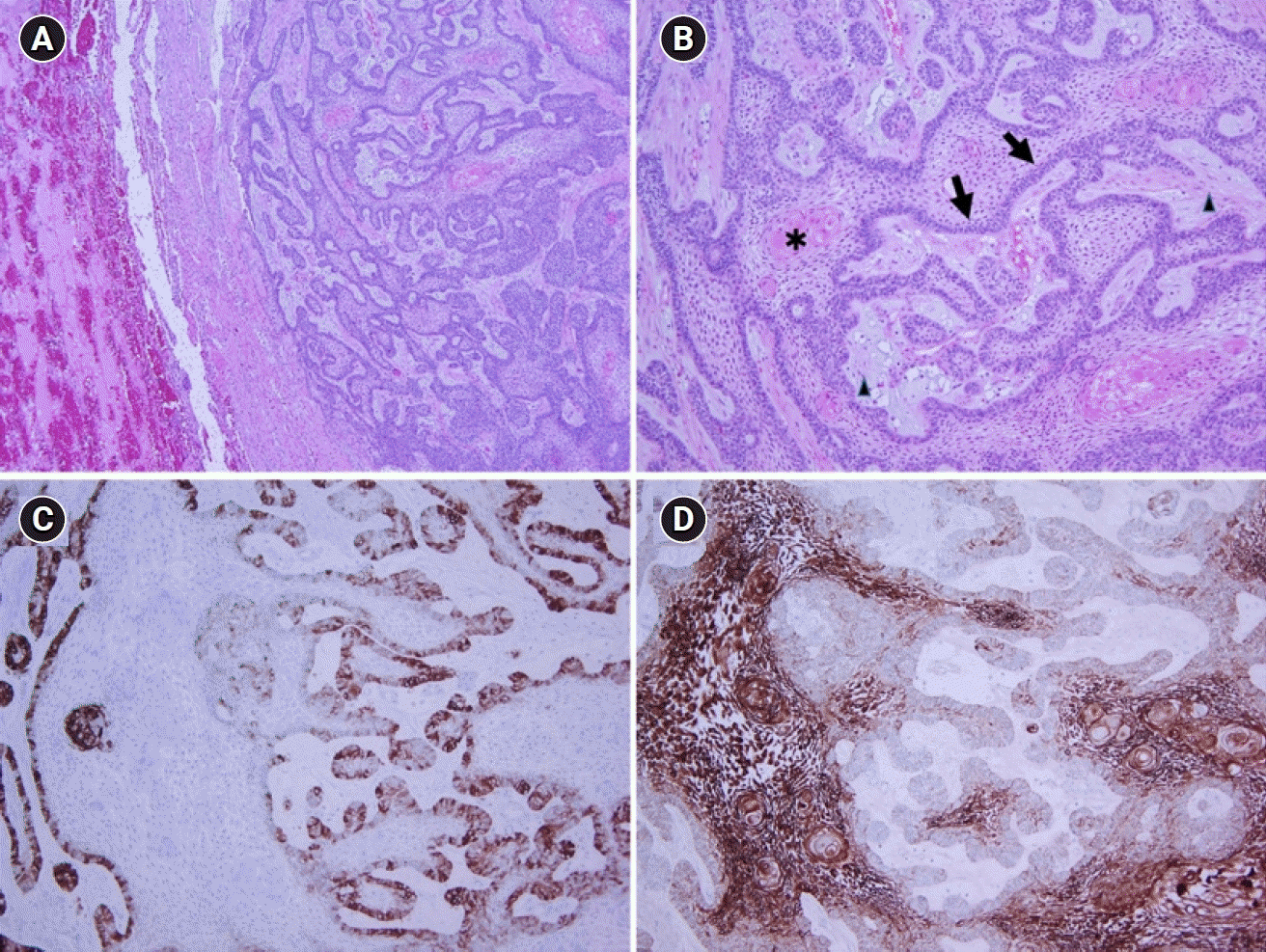
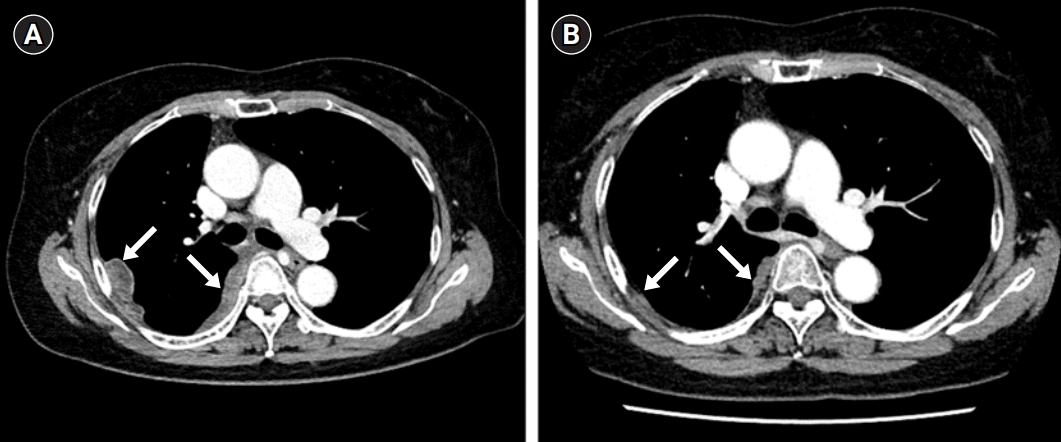
Fig. 4.
(A) Chest computed tomography performed in July 2020. Newly developed multifocal enhancing pleural and fissural nodules in the right hemithorax (arrows). (B) Follow-up chest computed tomography performed after chemotherapy, showing markedly decreased size of the previously noted pleural and fissural nodules in the right hemithorax (arrows).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download