Abstract
Background
The American Society of Anesthesiologists (ASA) score is generated based on patients’ clinical status. Accurate ASA classification is essential for the communication of perioperative risks and resource planning. Literature suggests that ASA classification can be automated for consistency and time-efficiency. To develop a rule-based algorithm for automated ASA classification, this study seeks to establish consensus in ASA classification for clinical conditions encountered at a tertiary women’s hospital.
Methods
Thirty-seven anesthesia providers rated their agreement on a 4-point Likert scale to ASA scores assigned to items via the Delphi technique. After Round 1, the group’s collective responses and individual item scores were shared with participants to improve their responses for Round 2. For each item, the percentage agreement (‘agree’ and ‘strongly agree’ responses combined), median (interquartile range/IQR), and SD were calculated. Consensus for each item was defined as a percentage agreement ≥ 70%, IQR ≤ 1.0, and SD < 1.0.
Results
All participants completed the study and none had missing data. The number of items that reached consensus increased from 25 (51.0%) to 37 (75.5%) in the second Delphi round, particularly for items assigned ASA scores of III and IV. Nine items, which pertained to alcohol intake, asthma, thyroid disease, limited exercise tolerance, and stable angina, did not reach consensus even after two Delphi rounds.
Pre-anesthesia assessment is the process of clinical evaluation that precedes the delivery of anesthesia for surgical and non-surgical procedures [1]. Upon completing this assessment, it is standard practice to assign an American Society of Anesthesiologists (ASA) score based on the patient’s clinical status [2]. The ASA classification system is most widely used in the pre-anesthesia assessment for surgical patients and aids in resource planning [3], reimbursement of anesthesia services [4] and prediction of complications [5].
Despite its widespread use, studies suggest poor inter-rater agreement on ASA classifications [6–10]. Interpretations of ASA definitions may be influenced by the patient case-mix [11,12], rater expertise [9,11], and healthcare funding model [6]. However, consistency in ASA classification is vital for accurate risk prediction and resource planning. With the establishment of outpatient pre-anesthesia evaluation clinics, discrepancies between the ASA classification assigned by preprocedural and day-of-surgery anesthesiologists could lead to day-of-surgery cancellations, which are associated with decreased operating room efficiency, low staff morale, increased patient anxiety, and increased costs [13,14].
Traditional models of in-person pre-anesthesia assessments have transitioned to digital formats, administered by health care providers or self-administered by patients [15–23]. Electronic pre-anesthesia assessment platforms often incorporate clinical decision support systems (CDSS) that improve quality of care through the standardization of practice [24,25]. We previously reported the development and validation of a web-based Pre-AnaesThesia Computerized Health (PATCH) assessment application through a mixed-methods approach [22]. The PATCH application allows patients to self-administer a pre-anesthesia health screening questionnaire on a mobile device at the time, place, and pace most convenient to them. Patient responses gathered online generate a comprehensive health report that is as reliable and accurate as that of nurse-led assessment [23]. However, in its current form, the application does not automatically generate the ASA score. Therefore, we aimed to build a CDSS for automated ASA classification for integration into the PATCH application.
As part of the ongoing research to develop a CDSS for automated ASA classification, the present study was undertaken with the aim of establishing Delphi consensus in ASA classification for a spectrum of clinical conditions encountered in our tertiary women’s hospital setting. As is typical of the Delphi technique, experts’ opinions were sought to determine the extent of agreement between them, and discrepancies were resolved through a series of anonymized sequential rounds, interspersed with controlled feedback and an opportunity for respondents to modify their responses [26]. Items that attained consensus could then be incorporated to build decision rules for the program algorithm to automate ASA classification.
Ethics approval of the study (2017/3002) was provided by the SingHealth Centralized Institutional Review Board of the Singapore Health Services Private Limited. The study was conducted at the Department of Women’s Anesthesia of the KK Women’s and Children’s Hospital, Singapore from 2 January to 28 February 2021. The 830-bed hospital provides tertiary care for women and children. Eligible experts contacted for the Delphi study were anesthesia providers of the department who staffed the outpatient pre-anesthesia evaluation clinics and operating rooms and had a minimum of two years’ experience providing supervised or independent anesthesia care. Purposive sampling was performed to ensure that the participants met eligibility criteria.
To assess Delphi consensus, two rounds of structured questionnaires were administered. Questionnaire items were formulated by three members of the study team (EL, BLS, and RD), each of whom have more than 20 years of clinical experience. The items covered patient conditions commonly encountered in our clinical setting and included examples adapted from the ASA-approved examples [2]. Conditions that are typically classified as ASA V (e.g., moribund patient) and VI (e.g., brain death) were excluded from the study, as they are not considered controversial in nature. The first version of the questionnaire was evaluated for clarity and relevance by two consultant anesthesiologists not affiliated with the hospital. No changes were deemed necessary after their review.
After providing written informed consent, participants accessed a web-based questionnaire
(https://form.gov.sg/5fb48cb93a3ec7001128173b) to rate their agreement (on a 4-point Likert scale) to ASA scores assigned to 49 items in the ASA questionnaire framework. The participants had the option to provide free-text comments on individual items. The participants were also asked to provide information on gender and clinical experience.
Participants were instructed to indicate their level of agreement on a 4-point Likert scale (strongly disagree ‘1’, disagree ‘2’, agree ‘3’, and strongly agree ‘4’) to ASA scores assigned to the 49 items. The ‘neutral’ option was removed to move the group towards consensus [27] and produce stable findings in the Delphi [28].
Four weeks after completion of the first Delphi round, participants received an individualized questionnaire in Excel format via email denoting their individual scores, the group median, distribution of responses, and the free-text comments collected in Round 1. Participants were then asked to reconsider their responses for Round 2, taking into consideration the group’s collective responses (i.e., median ASA score for each item) and comments obtained in Round 1. The method of providing feedback along with the distribution of responses per item has been previously described in similar Delphi studies [29]. For Round 2, participants were allowed to review their ratings in order to potentially achieve a level of consensus for the group rating. Free-text comments were not elicited for any of the items in Round 2. Fig. 1 summarizes the process of the Delphi technique used for this study.
We had aimed to conduct two Delphi rounds, making an a priori decision to proceed with Round 3 if consensus was not achieved by Round 2.
For the present study, consensus for each item was determined by a combination of the percentage agreement, interquartile range (IQR), and standard deviation (SD). Although using the percentage level setting based on the majority may be considered subjective [30], adding the IQR and SD increased the rigor regarding consensus since they are a measure of the stability of responses between rounds and level of convergence in the participants’ assessment [31,32].
To measure consensus in this study, the following criteria were used in combination a priori:
1. Percentage agreement ≥ 70%, meaning ≥ 70% of participants must either agree or strongly agree (Likert scale ≥ 3) with an item in Round 2 for it to be included in the ASA score assignment framework. This level of agreement has been described in previous studies using the Delphi technique [33].
2. IQR ≤ 1.0, meaning the IQR lies within one unit of the median on a 4-point Likert scale [31].
3. SD < 1.0, which indicates homogeneity in the participants’ responses [32].
Failure to achieve consensus in Round 2 on all three measures resulted in the item being excluded.
Data were analyzed using IBM SPSS Statistics for Windows (IBM Corp., USA) at the conclusion of each round. Demographic data and Likert item responses were analyzed using descriptive statistics. The median (IQR) score was calculated for each item. The categories ‘strongly agree’ and ‘agree’ were combined to compute the percentage agreement of each item. Variability in responses was measured using the SD, where a decrease in the SD between rounds indicated increasing homogeneity of the response. Regardless of whether the level of consensus was obtained in Round 1, all items were re-introduced in Round 2 of the Delphi survey to give every item the same opportunity to gain the highest rating and level of consensus.
All 37 eligible staff members of the anesthesia department (excluding the three study team members) consented to the study and completed both Delphi rounds with no missing data (100% response rate). Table 1 shows the demographic characteristics of the 37 participants, comprising 15 consultant anesthesiologists, two anesthesia nurse practitioners, 14 residents, and six resident physicians. The majority (75.7%) of participants had ≥ five years of experience in providing anesthesia care.
Tables 2–5 shows the Delphi consensus levels of items at the end of two rounds. The number of items that reached consensus increased from 25 (51.0%) in the first round to 37 (75.5%) in the second round. The greatest increase in consensus occurred for the items assigned ASA scores III and IV. Consensus was obtained for 77.3% of items assigned ASA III (Table 4) and 100% of items assigned ASA IV (Table 5). Three items (age > 75 years, disseminated intravascular coagulation, and obstetric hemorrhage with Hb < 6 g/dl) did not achieve consensus in one assigned class but achieved consensus when assigned another ASA class.
After two Delphi rounds, consensus was not achieved for nine items, which pertained to alcohol intake of 1–2 pints twice a week, asthma with monthly attacks managed on home therapy, thyroid disease, exercise tolerance of one flight of stairs, and stable angina. As consensus was attained for at least 75% of the items after round two, it was deemed unnecessary to proceed with another consensus round and the study was concluded.
Delphi consensus was attained for 37 of the 49 clinical items (75.5%), facilitating their inclusion in a rule-based clinical support system designed to automate the prediction of the ASA classification. We postulate that the moderate level of consensus obtained could reflect the similarity in training background among anesthesia providers at our setting of predominantly obstetric and gynecological cases. The literature also suggests an increased inter-rater agreement in ASA classification when raters share common training backgrounds and experience [11].
However, three clinical items (age > 75 years, disseminated intravascular coagulation [DIC], and obstetric hemorrhage with Hb < 6 g/dl) did not achieve consensus in one allocated ASA class but did in another class.
Age alone is not a criterion for ASA classification, although chronic diseases are more prevalent with advanced age. Advanced age is also a risk factor for increased morbidity and mortality. Technically, ASA classification should be based on the assessment of underlying organ function resulting from deterioration associated with age or disease and not simply by an age cut-off. However, anesthesiologists have been known to apply an ASA score of II to otherwise healthy patients based on an arbitrary age criterion that ranges from 60 to 75 years [34], which was confirmed by participants in this study.
DIC is a condition characterized by macro- and microvascular thrombosis and progressive consumption coagulopathy. In pregnancy, it can be triggered by placental abruption, placenta previa, amniotic fluid embolism, intrauterine death, eclampsia, and the hemolysis, elevated liver enzymes, low platelet count syndrome. The mortality rate for DIC is reported to be 20% to 50% [35]. Hence, it is not surprising that the consensus rating of ASA IV was attained for DIC in this study.
Obstetric hemorrhage is a leading cause of maternal mortality, accounting for 27% of all maternal deaths [36]. As our institution is an obstetric tertiary referral center, anesthesia providers have had first-hand experience managing life-threatening obstetric hemorrhages, including placenta accreta spectrum disorders [37]. We postulate that clinical experiences had likely influenced the group consensus of an ASA score of IV for acute obstetric hemorrhage complicated by severe anemia.
After both Delphi rounds, the nine items that did not achieve consensus in ASA rating were alcohol consumption of 1–2 pints twice a week, asthma with monthly attacks managed by home therapy, thyroid disease with and without thyroid storm, exercise tolerance of one flight of stairs, and stable angina.
Participants could not reach a consensus on whether to assign an ASA score of II or III for alcoholic consumption of 1–2 pints twice a week. Based on the latest ASA guidelines, ‘minimal alcohol intake’ is an example of ASA I while ‘social drinking’ is considered ASA II [2]. The ASA definitions do not define differential volumes and alcohol concentrations. However, the U.S. Department of Agriculture defines social drinking as limited to ≤ 2 drinks a day in men and ≤ 1 drink a day in women [38]. Accordingly, the intake of 1–2 pints of alcohol twice a week would be considered minimal and should warrant an ASA I classification. Our results suggest that participants were likely to be up-to-date with current guidelines on alcohol consumption and of the opinion that the consumption of 1–2 pints twice a week warranted an ASA I classification.
No consensus was achieved regarding an ASA II classification for a patient with asthma with monthly attacks that could be controlled by home therapy. The ASA definitions have previously been criticized for their subjective nature [6–9], and this is a case in point. ‘Asthma with exacerbation’ is an approved example for ASA III; however, it is vague and does not quantify frequency and severity, thus making it difficult to differentiate between ASA II and III. Therefore, participants likely had mixed opinions on whether to assign an ASA II or III classification, thus accounting for the results obtained.
The ASA classification does not provide approved examples of thyroid disease [2]. The item description, which states ‘active thyroid disease with abnormal levels of thyroid hormone,' is vague and does not provide details regarding the symptomatology or serum thyroid hormone levels. Without the benefit of clinical examination and laboratory thyroid measurements, we postulate that the majority of participants chose to adopt a more conservative approach in assigning ASA III to cases of active thyroid disease in the absence of thyroid storm. This failure to achieve consensus among participants could be explained by the fact that the presence of a thyroid storm is associated with a mortality of 10% [39], and an ASA IV classification would have been the appropriate option in that case.
Exercise tolerance is an important predictor of cardiovascular complications after non-cardiac surgery [40]. In the preoperative setting, exercise tolerance can be estimated from activities of daily living using metabolic equivalents (METs), where 1 MET is the resting oxygen consumption of a 40-year-old, 70 kg man [41,42]. Exercise tolerance for one flight of stairs or ≥ 4 METs [40] is usually used as a discriminator for further preoperative cardiac testing [41]. In the present study, participants agreed that an exercise capacity of one flight of stairs constituted an ASA III classification but could not agree that an exercise capacity of two flights of stairs constituted an ASA II physical status classification.
Stable angina is characterized by chest pain that is precipitated by exertion but relieved with rest or medication. In the ASA guidelines and approved examples [2], myocardial infarction is listed as an approved example, with onset ≤ 3 months as a discriminator between ASA III and ASA IV classifications. Besides this temporal relationship, stable and unstable angina are not provided as approved examples for ASA classification. Hence, participants likely drew upon their own varied clinical experience for interpretation, resulting in the lack of consensus.
The findings of this study provide a preliminary platform to establish decision ‘rules’ for the automated prediction of ASA classification scores, with the benefit of improved productivity and consistency in classification. A CDSS can either be knowledge-based and implemented as a conditional logic, or non-knowledge-based using artificial intelligence to derive patterns from clinical data sets [45]. CDSSs aid clinical decision making [46] and have been implemented for direct patient care [47] or to improve protocol compliance and quality measures [48]. More recently, CDSSs incorporating the automated prediction of a patient’s ASA classification have been reported [24,49]. In one study, data from a web-based preoperative assessment system were processed using decision logic to provide automated computation of ASA scores [24]. Except for 159 cases (or 1.1%), the computed ASA scores showed close agreement with ASA scores estimated clinically by a heterogeneous group of anesthesia providers. Machine learning approaches have also been developed to predict ASA classification [49]; however, the quality of the algorithm’s output is highly dependent on the quality and size of the data sets. A simple and basic CDSS based on the ‘IF THEN’ rule could be designed using data from the present study. For example, a patient aged > 75 years would automatically be assigned an ASA class of II based on the consensus attained, unless it is superseded by another condition that warrants a higher ASA classification score.
This study has a number of strengths and limitations. Although the sample size was only 37, a 100% response rate was obtained for both Delphi rounds. To ensure the robustness of the Delphi, all items in Round 1 were maintained in Round 2 to give every item an equal opportunity of attaining consensus in each round. The re-circulation of items also made it possible to compare the IQR, which indicated whether consensus was present throughout or only developed between rounds. However, the study was conducted at a single institution with its unique case-mix; therefore, external validity of the results is limited. The level of consensus could also vary in another population of anesthesia providers or even in the same population at another time. Additionally, controversial items could have been repeated under other ASA classes to give participants the chance to achieve consensus in these ASA classes. To develop an accurate and robust system for automated ASA classification, consensus should ideally be achieved for all items. This can be achieved by training participants in ASA classification. Future research should also evaluate consensus on a wider range of clinical conditions (including clinical and laboratory data) to improve the internal validity of the system. Consensus could also be evaluated through clinical vignettes oriented to local practice, as this has been shown to improve the internal consistency of ASA classifications [50].
In the present study, Delphi consensus in ASA classification was attained for 37 of the 49 (75.5%) example cases commonly encountered at our tertiary women’s hospital. This facilitated the development of a rule-based CDSS for the automated prediction of ASA classification in a pre-anesthesia health assessment application. Future research should seek consensus in ASA classification on a wider range of clinical conditions and vignettes to improve internal validity.
ACKNOWLEDGMENTS
The authors would like to thank Agnes Teo for managing the administrative activities related to the conduct of the study.
Notes
Author Contributions
Tarig Osman (Conceptualization; Formal analysis; Writing – original draft)
Eileen Lew (Conceptualization; Data curation; Funding acquisition; Writing – review & editing)
Ban L. Sng (Data curation; Writing – review & editing)
Rajive Dabas (Data curation; Writing – review & editing)
Konstadina Griva (Formal analysis; Writing – review & editing)
Josip Car (Writing – review & editing)
References
1. Pasternak LR, Arens JF, Caplan RA, Connis RT, Fleisher LA, Flowerdew R, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2012; 116:522–38.
2. American Society of Anesthesiologists. ASA physical status classification system [Internet]. Schaumburg (IL): ASA;2014. Oct 15 [updated 2020 Dec 13; cited 2021 Oct 13]. Available from https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system.
3. De Cassai A, Boscolo A, Tonetti T, Ban I, Ori C. Assignment of ASA-physical status relates to anesthesiologists’ experience: a survey-based national-study. Korean J Anesthesiol. 2019; 72:53–9.

4. Enneking FK, Radhakrishnan NS, Berg K, Patel S, Wishin JM, Vasilopoulos T. Patient-centered anesthesia triage system predicts ASA physical status. Anesth Analg. 2017; 124:1957–62.

5. Mak PH, Campbell RC, Irwin MG; American Society of Anesthesiologists. The ASA Physical Status Classification: inter-observer consistency. American Society of Anesthesiologists. Anaesth Intensive Care. 2002; 30:633–40.

6. Owens WD, Felts JA, Spitznagel EL Jr. ASA physical status classifications: a study of consistency of ratings. Anesthesiology. 1978; 49:239–43.
7. Haynes SR, Lawler PG. An assessment of the consistency of ASA physical status classification allocation. Anaesthesia. 1995; 50:195–9.

8. Ranta S, Hynynen M, Tammisto T. A survey of the ASA physical status classification: significant variation in allocation among Finnish anaesthesiologists. Acta Anaesthesiol Scand. 1997; 41:629–32.

9. Ihejirika RC, Thakore RV, Sathiyakumar V, Ehrenfeld JM, Obremskey WT, Sethi MK. An assessment of the inter-rater reliability of the ASA physical status score in the orthopaedic trauma population. Injury. 2015; 46:542–6.

10. Kuza CM, Hatzakis G, Nahmias JT. The Assignment of American Society of Anesthesiologists physical status classification for adult polytrauma patients: results from a survey and future considerations. Anesth Analg. 2017; 125:1960–6.
11. Jacqueline R, Malviya S, Burke C, Reynolds P. An assessment of interrater reliability of the ASA physical status classification in pediatric surgical patients. Paediatr Anaesth. 2006; 16:928–31.

12. Tait AR, Voepel-Lewis T, Munro HM, Gutstein HB, Reynolds PI. Cancellation of pediatric outpatient surgery: economic and emotional implications for patients and their families. J Clin Anesth. 1997; 9:213–9.

13. Van Klei WA, Moons KG, Rutten CL, Schuurhuis A, Knape JT, Kalkman CJ, et al. The effect of outpatient preoperative evaluation of hospital inpatients on cancellation of surgery and length of hospital stay. Anesth Analg. 2002; 94:644–9.

14. Goodhart IM, Andrzejowski JC, Jones GL, Berthoud M, Dennis A, Mills GH, et al. Patient-completed, preoperative web-based anaesthetic assessment questionnaire (Electronic Personal Assessment Questionnaire PreOperative): development and validation. Eur J Anaesthesiol. 2017; 34:221–8.
15. Vitkun SA, Gage JS, Anderson DH 2nd, Williams SA, Halpern-Lewis JG, Poppers PJ. Computerization of the preoperative anesthesia interview. Int J Clin Monit Comput. 1995; 12:71–6.

16. VanDenKerkhof EG, Goldstein DH, Blaine WC, Rimmer MJ. A comparison of paper with electronic patient-completed questionnaires in a preoperative clinic. Anesth Analg. 2005; 101:1075–80.

17. Tompkins BM, Tompkins WJ, Loder E, Noonan AF. A computer-assisted preanesthesia interview: value of a computer-generated summary of patient's historical information in the preanesthesia visit. Anesth Analg. 1980; 59:3–10.
18. Essin DJ, Dishakjian R, deCiutiis VL, Essin CD, Steen SN. Development and assessment of a computer-based preanesthetic patient evaluation system for obstetrical anesthesia. J Clin Monit Comput. 1998; 14:95–100.
19. Beers RA, O’Leary CE, Franklin PD. Comparing the history-taking methods used during a preanesthesia visit: the HealthQuiz versus the written questionnaire. Anesth Analg. 1998; 86:134–7.
20. Howell M, Hood AJ, Jayne DG. Use of a patient completed iPad questionnaire to improve pre-operative assessment. J Clin Monit Comput. 2017; 31:221–5.

21. Osman T, Lew E, Lum EP, van Galen L, Dabas R, Sng BL, et al. PreAnaesThesia computerized health (PATCH) assessment: development and validation. BMC Anesthesiol. 2020; 20:286.

22. Osman T, Lew E, Lum E, Chew J, Dabas R, Sng BL, et al. Effect of PreAnaesThesia Computerized Health (PATCH) assessment on duration of nurse-patient consultation and patient experience: a pilot trial. Int J Environ Res Public Health. 2020; 17:4972.

23. Zuidema X, Tromp Meesters RC, Siccama I, Houweling PL. Computerized model for preoperative risk assessment. Br J Anaesth. 2011; 107:180–5.

24. Sim EY, Tan DJ, Abdullah HR. The use of computerized physician order entry with clinical decision support reduces practice variance in ordering preoperative investigations: a retrospective cohort study. Int J Med Inform. 2017; 108:29–35.

25. Jones J, Hunter D. Consensus methods for medical and health services research. BMJ. 1995; 311:376–80.

26. McPherson S, Reese C, Wendler MC. Methodology update: Delphi studies. Nurs Res. 2018; 67:404–10.
27. Vogel C, Zwolinsky S, Griffiths C, Hobbs M, Henderson E, Wilkins E. A Delphi study to build consensus on the definition and use of big data in obesity research. Int J Obes (Lond). 2019; 43:2573–86.

28. MacLennan S, Kirkham J, Lam TB, Williamson PR. A randomized trial comparing three Delphi feedback strategies found no evidence of a difference in a setting with high initial agreement. J Clin Epidemiol. 2018; 93:1–8.

29. Brunt H, Barnes J, Longhurst JW, Scally G, Hayes E. Enhancing local air quality management to maximise public health integration, collaboration and impact in Wales, UK: a Delphi study. Environ Sci Policy. 2018; 80:105–16.

30. Heiko A, von der Gracht. Consensus measurement in Delphi studies: review and implications for future quality assurance. Technol Forecast Soc Change. 2012; 79:1525–36.
31. Schmalz U, Spinler S, Ringbeck J. Lessons learned from a two-round Delphi-based scenario study. MethodsX. 2020; 8:101179.

32. Hsu CC, Sandford BA. The Delphi technique: making sense of consensus. Pract Assess Res Eval. 2007; 12:10.
33. Akhtar S, Barker SJ, Bose R, Botts A, Burstrom RE, Culley DJ, et al. Frequently asked questions: about anesthetic considerations for elderly patients [Internet]. Park Ridge: American Society of Anesthesiologists. 2009;[cited 2021 Oct 13]. Available from www.sagahq.org/images/FAQs.pdf.
34. Gando S, Saitoh D, Ogura H, Mayumi T, Koseki K, Ikeda T, et al. Natural history of disseminated intravascular coagulation diagnosed based on the newly established diagnostic criteria for critically ill patients: results of a multicenter, prospective survey. Crit Care Med. 2008; 36:145–50.

35. Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014; 2:e323–33.

36. Lional KM, Tagore S, Wright AM. Uterine conservation in placenta accrete spectrum (PAS) disorders: a retrospective case series: is expectant management beneficial in reducing maternal morbidity? Eur J Obstet Gynecol Reprod Biol. 2020; 254:212–7.
37. U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary guidelines for Americans, 2020-2025 [Internet]. Washington: USDA Publication. 2020 Dec [cited 2021 Oct 13]. Available from www.DietaryGuidelines.gov.
38. Chiha M, Samarasinghe S, Kabaker AS. Thyroid storm: an updated review. J Intensive Care Med. 2015; 30:131–40.
39. Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014; 130:2215–45.

40. Reilly DF, McNeely MJ, Doerner D, Greenberg DL, Staiger TO, Geist MJ, et al. Self-reported exercise tolerance and the risk of serious perioperative complications. Arch Intern Med. 1999; 159:2185–92.

41. Fleg JL, Piña IL, Balady GJ, Chaitman BR, Fletcher B, Lavie C, et al. Assessment of functional capacity in clinical and research applications: an advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association. Circulation. 2000; 102:1591–7.

42. Biccard BM. Relationship between the inability to climb two flights of stairs and outcome after major non-cardiac surgery: implications for the pre-operative assessment of functional capacity. Anaesthesia. 2005; 60:588–93.

43. Dosluoglu HH, Wang J, Defranks-Anain L, Rainstein M, Nader ND. A simple subclassification of American Society of Anesthesiology III patients undergoing peripheral revascularization based on functional capacity. J Vasc Surg. 2008; 47:766–72.

44. Sutton RT, Pincock D, Baumgart DC, Sadowski DC, Fedorak RN, Kroeker KI. An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit Med. 2020; 3:17.

45. Zikos D, DeLellis N. CDSS-RM: a clinical decision support system reference model. BMC Med Res Methodol. 2018; 18:137.

46. Eden A, Pizov R, Toderis L, Kantor G, Perel A. The impact of an electronic reminder on the use of alarms after separation from cardiopulmonary bypass. Anesth Analg. 2009; 108:1203–8.
47. McDonald CJ. Protocol-based computer reminders, the quality of care and the non-perfectability of man. N Engl J Med. 1976; 295:1351–5.

48. Lazouni ME, Daho ME, Settouti N, Chikh MA, Mahmoudi S. Machine learning tool for automatic ASA detection. In: Modeling Approaches and Algorithms for Advanced Computer Applications. Amine A, Otmane AM, Bellatreche L, editors. Cham: Springer International Publishing;2013. p. 9–16.
49. Hurwitz EE, Simon M, Vinta SR, Zehm CF, Shabot SM, Minhajuddin A, et al. Adding examples to the ASA-Physical Status classification improves correct assignment to patients. Anesthesiology. 2017; 126:614–22.

50. Aronson WL, McAuliffe MS, Miller K. Variability in the American Society of Anesthesiologists Physical Status Classification scale. AANA J. 2003; 71:265–74.
Fig. 1.
Flow diagram illustrating the Delphi method used. ASA: American Society of Anesthesiologists, IQR: interquartile range, SD: standard deviation.
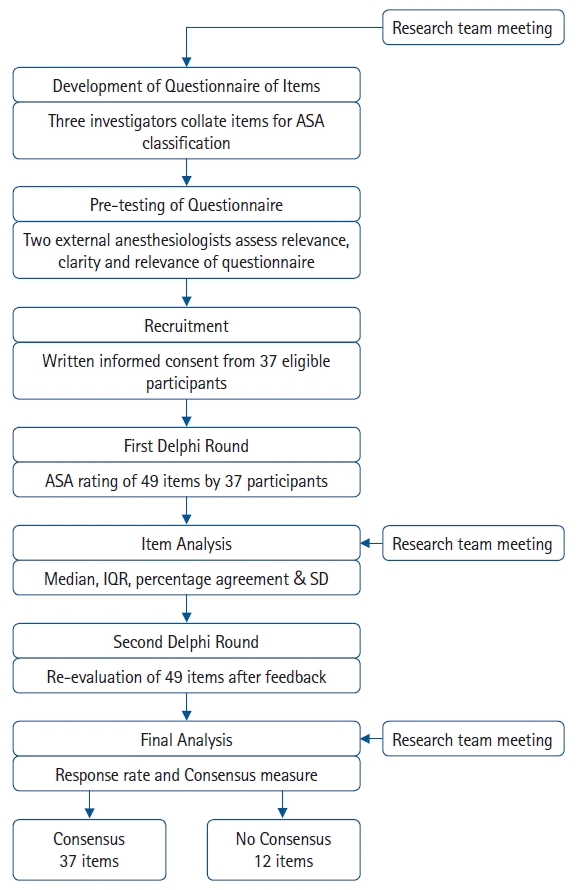
Table 1.
Demographic Characteristics of Respondents (n = 37)
| Frequency | Percent | |
|---|---|---|
| Sex (M/F) | 17/20 | 45.9/54.1 |
| Job position | ||
| Consultants | 15 | 43.2 |
| Non-consultants | 22 | 56.8 |
| Years of experience | ||
| 1 to < 5 | 9 | 24.3 |
| ≥ 5 | 28 | 75.7 |
Table 2.
Consensus Levels Achieved for Clinical Items Assigned an ASA I Score
| Items |
Round 1 |
Round 2 |
Outcome‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ASA I | PA* | Median† | IQR | SD | PA* | Median† | IQR | SD | |
| Age > 75 yr | 59.5 | 3 | 1.0 | 0.90 | 54.1 | 3 | 1.0 | 0.80 | No |
| No or minimal alcohol use | 100 | 4 | 1.0 | 0.48 | 94.6 | 4 | 1.0 | 0.58 | Yes |
| BMI 28 | 83.8 | 3 | 1.0 | 0.79 | 70.3 | 3 | 1.0 | 0.82 | Yes |
Table 3.
Consensus Levels Achieved for Clinical Items Assigned an ASA II Score
| Items |
Round 1 |
Round 2 |
Outcome‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ASA II | PA* | Median† | IQR | SD | PA* | Median† | IQR | SD | ||
| Current smoker of 10 pack-years | 86.5 | 3 | 1.0 | 0.69 | 81.1 | 3 | 1.0 | 0.70 | Yes | |
| Alcohol intake of 1–2 pints twice a week | 56.8 | 3 | 1.0 | 0.79 | 62.2 | 3 | 1.0 | 0.62 | No | |
| Pregnancy with twins | 81.1 | 3 | 1.0 | 0.76 | 86.5 | 3 | 0 | 0.60 | Yes | |
| Obesity with BMI 32 | 81.1 | 3 | 1.0 | 0.71 | 78.4 | 3 | 0 | 0.71 | Yes | |
| Chronic schizophrenia, on medications | 89.2 | 3 | 1.0 | 0.65 | 89.2 | 3 | 1.0 | 0.67 | Yes | |
| Diabetes and HbA1c 6.5% | 97.3 | 3 | 1.0 | 0.56 | 94.6 | 3 | 1.0 | 0.57 | Yes | |
| Hypertension and BP readings < 150/90 mmHg | 89.2 | 3 | 1.0 | 0.71 | 94.6 | 3 | 1.0 | 0.57 | Yes | |
| Asthma with attacks once a month managed by home therapy | 75.7 | 3 | 1.5 | 0.87 | 73.0 | 3 | 1.5 | 0.83 | No | |
| Anemia with Hb 10 g/dl | 97.3 | 3 | 1.0 | 0.51 | 91.9 | 3 | 0.5 | 0.55 | Yes | |
| Aged > 75 yr | 59.5 | 3 | 1.0 | 0.66 | 75.7 | 3 | 0.5 | 0.55 | Yes | |
| Exercise tolerance of 2 flights of stairs | 45.9 | 3 | 2.0 | 1.11 | 21.6 | 2 | 0 | 0.60 | No | |
| Obstructive sleep apnea with STOP BANG score of 3 | 78.4 | 3 | 0 | 0.66 | 81.1 | 3 | 0 | 0.48 | Yes | |
| Active thyroid disease with abnormal levels of free thyroxine but not in thyroid storm | 45.9 | 2 | 1.0 | 0.87 | 27 | 2 | 1.0 | 0.57 | No | |
| Disseminated intravascular coagulation | 2.7 | 1 | 1.0 | 0.51 | 2.7 | 1 | 0.5 | 0.51 | No | |
| Obstetric hemorrhage with Hb 6 g/dl | 16.2 | 1 | 1.0 | 0.55 | 2.7 | 1 | 1.0 | 0.55 | No | |
Table 4.
Consensus Levels Achieved for Clinical Items Assigned an ASA III Score
| Items |
Round 1 |
Round 2 |
Outcome‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ASA III | PA* | Median† | IQR | SD | PA* | Median† | IQR | SD | |
| Poorly controlled diabetes with HbA1c 10% | 91.9 | 3 | 1.0 | 0.72 | 97.3 | 3 | 1.0 | 0.61 | Yes |
| Hypertension and BP readings 160/105 mmHg | 91.9 | 3 | 1.0 | 0.72 | 100 | 3 | 1.0 | 0.45 | Yes |
| Chronic obstructive lung disease with daily exacerbations | 62.2 | 3 | 2.0 | 1.1 | 78.4 | 3 | 1.0 | 0.88 | Yes |
| BMI 42 | 89.2 | 4 | 1.0 | 0.77 | 94.6 | 4 | 1.0 | 0.58 | Yes |
| BMI 38 | 59.5 | 3 | 1.5 | 0.85 | 75.7 | 3 | 0.5 | 0.72 | Yes |
| Active hepatitis by clinical presentation and diagnostic results | 70.3 | 3 | 2.0 | 0.8 | 81.1 | 3 | 0 | 0.65 | Yes |
| Effort tolerance of one flight of stairs | 64.9 | 3 | 1.0 | 0.69 | 78.4 | 3 | 0 | 0.42 | Yes |
| Atrial fibrillation, rate 150 bpm | 64.9 | 3 | 2.0 | 1.04 | 75.7 | 3 | 0.5 | 0.85 | Yes |
| Myocardial ejection fraction 40% | 83.8 | 3 | 1.0 | 0.71 | 97.3 | 3 | 0 | 0.46 | Yes |
| End stage renal disease undergoing regularly scheduled peritoneal dialysis | 56.5 | 3 | 1.0 | 0.86 | 94.6 | 3 | 0 | 0.59 | Yes |
| End stage renal disease undergoing regularly scheduled hemodialysis | 86.5 | 3 | 1.0 | 0.88 | 94.6 | 3 | 0 | 0.57 | Yes |
| Myocardial infarct 6 months ago | 83.8 | 3 | 1.0 | 0.69 | 91.9 | 3 | 0 | 0.52 | Yes |
| Cerebrovascular accident or transient ischemic attack 4 months ago | 78.4 | 3 | 1.0 | 0.8 | 86.5 | 3 | 0.5 | 0.68 | Yes |
| Coronary stenting 6 months ago | 86.5 | 3 | 1.0 | 0.63 | 94.6 | 3 | 0 | 0.46 | Yes |
| Implanted pacemaker | 83.8 | 3 | 1.0 | 0.79 | 97.3 | 3 | 0 | 0.44 | Yes |
| Chest pain exacerbated by exertion and resolved with rest | 51.4 | 3 | 1.5 | 0.83 | 51.4 | 3 | 1.0 | 0.68 | No |
| Mitral stenosis with valve area 1.5 cm2 | 73 | 3 | 1.0 | 0.66 | 81.1 | 3 | 0 | 0.58 | Yes |
| Obstructive sleep apnea with STOP BANG score 5–6 | 83.8 | 3 | 1.0 | 0.76 | 86.5 | 3 | 0 | 0.65 | Yes |
| Active thyroid disease with abnormal levels of free thyroxine and in thyroid storm | 27.0 | 2 | 2.0 | 1.04 | 24.3 | 2 | 1.5 | 0.97 | No |
| Disseminated intravascular coagulation | 40.5 | 2 | 2.0 | 1.07 | 27.0 | 2 | 1.5 | 1.00 | No |
| Obstetric hemorrhage with Hb 6 g/dl | 35.1 | 2 | 1.0 | 0.96 | 29.7 | 2 | 1.0 | 0.88 | No |
| Alcohol intake of > 1–2 pints twice a week (1 pint = 500 ml) | 32.4 | 2 | 1.0 | 0.62 | 24.3 | 2 | 0.5 | 0.64 | No |
Table 5.
Consensus Levels Achieved for Clinical Items Assigned an ASA IV Score
| Items |
Round 1 |
Round 2 |
Outcome‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ASA IV | PA* | Median† | IQR | SD | PA* | Median† | IQR | SD | |
| Atrial fibrillation, rate 180 bpm | 100 | 4 | 1.0 | 0.48 | 100 | 4 | 0 | 0.40 | Yes |
| Myocardial infarct 2 months ago | 75.7 | 3 | 1.5 | 0.83 | 89.2 | 4 | 1.0 | 0.65 | Yes |
| Cerebrovascular accident or transient ischemic attack 3 months ago | 64.9 | 3 | 2.0 | 0.91 | 83.8 | 3 | 1.0 | 0.66 | Yes |
| Coronary stenting 2 months ago | 67.6 | 3 | 2.0 | 0.88 | 81.1 | 3 | 0 | 0.65 | Yes |
| Effort tolerance ≤ 1 flight of stairs | 59.5 | 3 | 1.0 | 0.82 | 70.3 | 3 | 1.0 | 0.65 | Yes |
| Mitral stenosis with valve area 0.8 cm2 | 81.1 | 3 | 1.0 | 0.79 | 89.2 | 3 | 0 | 0.57 | Yes |
| Myocardial ejection fraction 20% | 83.8 | 4 | 1.0 | 0.76 | 91.9 | 3 | 1.0 | 0.63 | Yes |
| Disseminated intravascular coagulation | 94.6 | 4 | 1.0 | 0.61 | 100 | 4 | 1.0 | 0.45 | Yes |
| Obstetric hemorrhage with Hb 6 g/dl | 73.0 | 3 | 2.0 | 0.89 | 84.8 | 3 | 1.0 | 0.99 | Yes |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download