Introduction
Materials and Methods
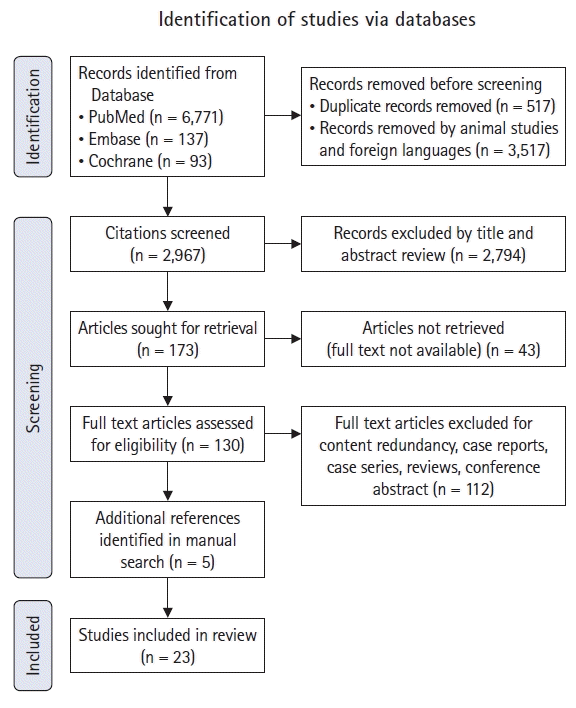
Study design
Literature search strategy
Table 1.
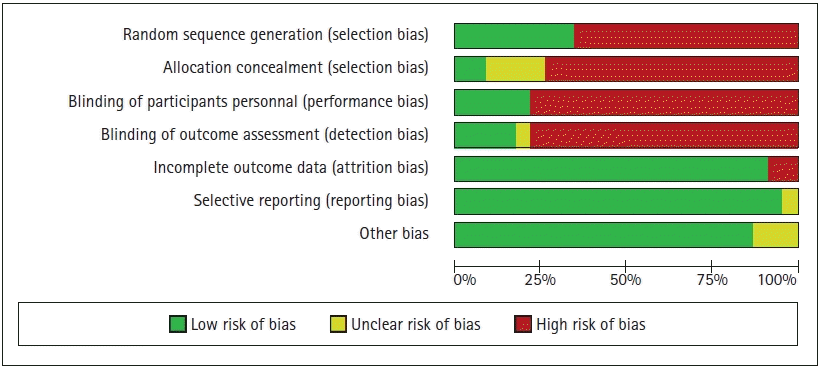
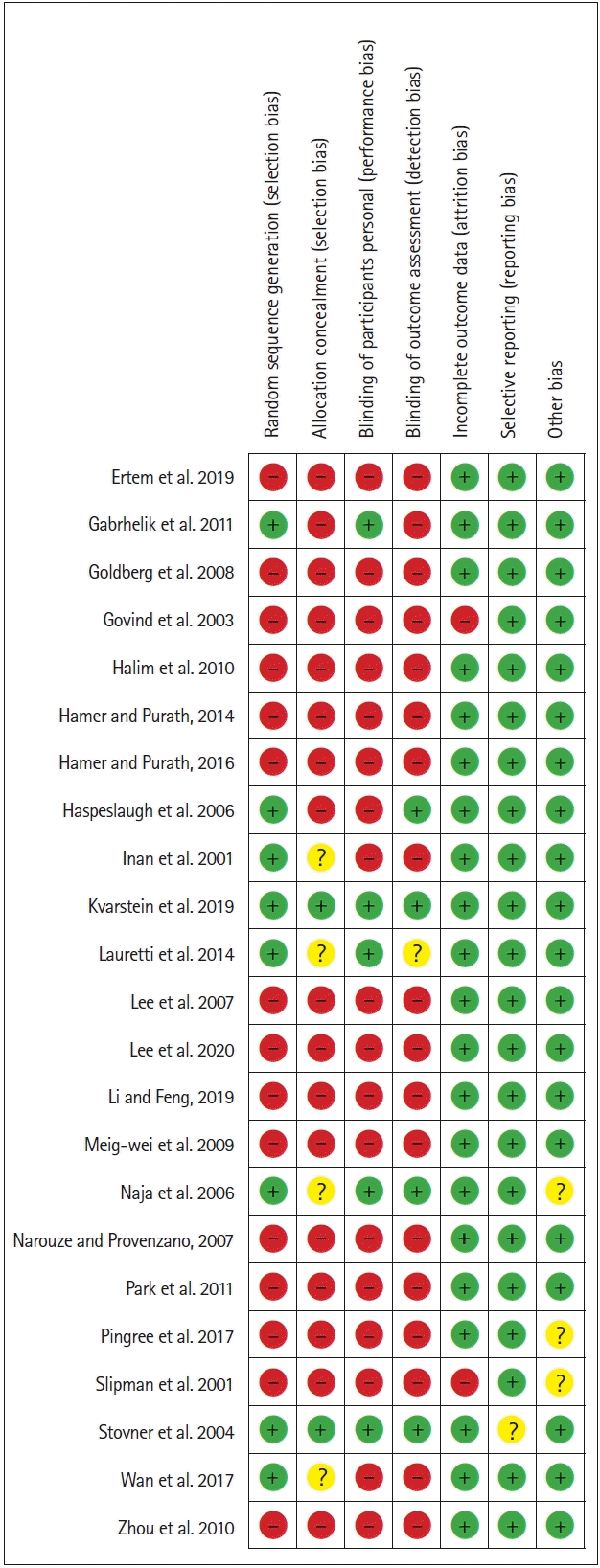
Assessment of risk of bias in individual studies
Data extraction
Results
 | Fig. 4.Study interventions included in this review. Of the 23 included studies, 11 studies evaluated the effect of RFA, 5 evaluated the role of occipital nerve blocks, 2 studied facet joint injections, 2 studied deep cervical plexus blocks, and 1 each studied AA joint injections, cervical epidural steroid injections, and cryoneurolysis on CeH. GON: greater occipital nerve, LON: lesser occipital nerve, AA: atlantoaxial, RFA: radiofrequency ablation, CeH: cervicogenic headache. |
Table 2.
| Article (yr) | Study type | Participants | Intervention | Results | Conclusion |
|---|---|---|---|---|---|
| Occipital nerve blocks (GON, LON) | |||||
| Inan et al. 2001 [14] | Randomized prospective comparative study | 28 patients with CeH based on diagnostic criteria by Sjaastad et al. | GON block group and C2/C3 nerve block group (1% lidocaine diagnostic block followed by two weekly injections of 0.25% bupivacaine) | Decreased pain frequency/duration in both groups lasting at least 2 months, with no significant group differences except pain frequency in first week after first therapeutic block (significantly lower in C2/C3 group) | Both blocks are equally effective for diagnosis and treatment of CeH |
| Naja et al. 2006 [9] | Double blind, placebo controlled | 50 (25 target, 25 control) patients with CeH | GON and LON blocks with or without facial nerve block (16/25); anesthetic block group compared with placebo group | Significant pain improvement, decreased analgesic use, and decreased duration/frequency of headache at 2 weeks | Nerve stimulator- guided occipital nerve block provides relief of pain and accompanying symptoms for up to 2 weeks |
| Lauretti et al. 2014 [15] | Randomized double-blinded | 30 patients with unilateral cervical pain with most painful point located on ipsilateral GON | GON block performed using classic technique followed by sub-compartmental technique if VAS > 3; final volume of 5, 10, or 15 ml (10 mg dexamethasone + 40 mg lidocaine + nonionic iodine contrast + saline) | Significant decrease in VAS and rescue analgesic consumption in all subcompartmental groups lasting 24 weeks compared to only 2 weeks for classic technique | -Classic technique resulted in only 2 weeks of analgesia whereas sub-compartmental resulted in at least 24 weeks of analgesia; |
| -5 ml volume sufficient for successful block | |||||
| Pingree et al. 2017 [16] | Prospective open label | 14 patients with occipital neuralgia or CeH | US-guided GON block at C2 level, 4 ml [1 ml of 2% lidocaine + 2.5 ml of 0.25% bupivacaine + 3 mg betamethasone] injected | -Successful block in 86% of patients. | -Successful blockade of GON at C2 using US-guided technique |
| -Significant decrease in mean NRS from 4.71 (baseline) to 3.78 at 30 minutes, 2.64 at 2 weeks, and 2.21 at 4 weeks | -Significant reduction in pain scores observed over 4-week period | ||||
| -No significant adverse effects reported | |||||
| Ertem and Yılmaz, 2019 [17] | Retrospective cohort study | 21 patients with CeH who underwent at least 3 GON blocks, attended at least 3 follow-up appointments, and were admitted to the headache clinic during a 6-month period | GON block at the scalp; injection mixture of 3–4 ml of 2% lidocaine + 1 ml methylprednisolone | -Significant decline in mean NRS by first month (second injection), second month (third injection), and third month (fourth injection) | -Repeat GON injections is an effective option in patients not responding to conservative therapy |
| -8 patients reported no pain after the second injection and thus did not receive a fourth injection | -No serious complication was noted | ||||
| Cervical facet joint injections | |||||
| Slipman et al. 2001 [18] | Retrospective | -18 patients with unremitting daily headaches after flexion/extension injury of upper cervical spine with tender facet joint not responding to conservative therapy | -C2–C3 facet joint diagnostic block followed by therapeutic injection if decrease in VAS was > 80% | -Average decrease in VAS (from 8.2 to 5.5) | Intraarticular facet joint injection is effective for treating headaches emanating from the C2–C3 joint after whiplash event |
| -Symptom duration ~34 months | -If symptom relief was < 90%, second therapeutic injection given after 2 weeks | -50% of patients experienced headache < 3 times/month, and 61% experienced < 3 episodes/week responsive to oral analgesics | |||
| -Follow up at ~19 months | |||||
| Zhou et al. 2010 [19] | Retrospective observational | 31 patients who failed multiple pharmacological/ other treatments | C1/2, C2/3 facet joint block and C2 and C3 spinal rami blocks using 0.5 ml 0.25% bupivacaine + 3 mg betamethasone | > 50% headache relief in 90.3% (28) of patients immediately after procedure; however, 9.7% (3) of patients did not respond | C1/C2 and C2/C3 facet joint and spinal rami blocks provide significant prolonged pain relief in > 90% of patients |
| AA joint intraarticular injection | |||||
| Narouze and Provenzano, 2007 [20] | Retrospective | 32 patients with clinical picture suggestive of AA joint pain, intractable headaches, and failed multiple drug treatment | Classic intraarticular posterior approach, lateral AA joint injection (1 ml bupivacaine 0.5% + 10 mg triamcinolone) | -Post-procedure pain score was 0 in 46.8% of patients (15); ≥ 50% decrease in pain score in 81.2% of patients (23) | Lateral AA intraarticular steroid injections provide short-term analgesia |
| -Significant decrease in pain score at 1 and 3 months but not at 6 months | |||||
| Deep cervical plexus block | |||||
| Goldberg et al. 2008 [21] | Prospective | 39 patients with CeH | -Deep cervical plexus block @ C2/C3 using 10 ml 0.25% bupivacaine + 80 mg methylprednisolone | -Significant decrease in pain scores (P < 0.001) | -Significant decrease in pain after initial as well as last treatment |
| -For unilateral headache, unilateral block given and repeated on contralateral side after 1 week for global headache | -33% of patients reported pain scores ≤ 4 after their last treatment | -For some patients, effective pain relief was seen for 3 months but by 6 months, pain had returned to pre-treatment levels | |||
| -Pain assessed pre- and immediate post-injection and at 3 and 6 months | -24% (10) had pain scores ≤ 4 at 3 months and 18% (7) had pain scores ≤ 4 at 6 months | ||||
| Wan et al. 2017 [22] | RCT; single-blinded | 56 patients with CeH randomly recruited to either US-guided or FL-guided injection group | Mixture of 2–4 ml 1% lidocaine + 7 mg betamethasone injected along C2 and/or C3 transverse process | -Significant decrease in NRS in both groups (P < 0.05) at 2, 12, and 24 weeks post-injection | -DCP block provides significant pain relief (for up to 6 months) |
| -No serious side effects reported | -US-guided approach showed similar satisfactory effect as FL-guided with advantage of no radiation exposure | ||||
| Continuous cervical epidural block | |||||
| He et al., 2009 [23] | Retrospective observational | 37 patients with CeH | Epidural catheter placed in C6–C7, C7–T1 or T1–T2 space; lidocaine (100–200 mg) + dexa. (1–2 mg) + saline (total 250 ml) infused @ 5 ml/hr for 3–4 weeks. In addition, 5 mg triamcinolone given once weekly for 3–4 weeks, then catheters removed | Days with mild/moderate pain, occurrence of severe pain, and daily NSAID dose (mg) significantly reduced 6 months after catheter placement compared to 3 months prior to procedure | -Effective for at least six months. |
| -Further research is needed to elucidate mechanisms of action and to prolong this effect | |||||
| Radiofrequency ablation (RFA) | |||||
| RFA of facet joint and medial branches supplying the facet joint | |||||
| Stovner et al. 2004 [25] | Randomized, double-blind | 12 patients with refractory CeH randomized into RFN of C2–C6 facet joints vs. sham treatment | RFA of medial branch of C2–C6 facet joints on the symptomatic side | Slight improvement was seen at 3 months in RFN group, but no significant difference was seen after 3 months | -No benefit with the procedure |
| -Minor and short-term side-effects were seen | |||||
| Haspeslagh et al. 2006 [26] | Randomized, controlled | 30 patients with CeH randomized into two groups, the RFA group (n=15) and the LA group (n=15) | RFA of medial branches of dorsal rami of C3–C4 facet joint vs. LA with steroid injection at GON, followed by TENS when necessary | No statistically significant differences in pain scores | No evidence that RFN of cervical facet joints is a better treatment than infiltration of GON followed by TENS |
| Govind et al. 2003 [27] | Prospective, non-randomized | 49 patients suffering from third occipital nerve headache | RFN of third occipital nerve (medial branch of C3 spinal nerve, supplying C2–C3 facet joint) | -88% achieved a successful outcome | Profound pain relief was seen and repeat ablation prolonged the duration of pain relief |
| -Median duration of relief was around 297 days | |||||
| Lee et al. 2007 [28] | Prospective observational | 30 patients suffering from chronic CeH for > 6 months, with > 50% pain relief from two diagnostic C3–C4 cervical medial branch blocks | RFN of medial branch of C3–C4 facet joint | -Significantly reduced headache severity in 73% of patients at 12 months | -Substantial pain relief was seen |
| -75% pain relief seen in majority of patients | -No major complications reported | ||||
| -Reduced analgesic intake by 70% | |||||
| -Average headache episode decreased from 6.2 to 2.8 days/week | |||||
| Park et al. 2011 [29] | Retrospective observational | 11 patients with CeH | RFN of lower cervical medial branches (C4–C7) | -VAS score decreased by around 60% at 6 months | Lower cervical disorders can also lead to headaches, which can be improved with RFN |
| -No serious complication was reported | |||||
| RFA of GON | |||||
| Gabrhelík et al. 2011 [30] | Randomized clinical pilot study | 30 patients with refractory CeH randomized into two groups: the LA group (n=15) and the PRF group (n=15) | GON block with LA and steroid compared to PRF of GON | -Significant decrease in VAS seen in both groups at 3 months | -PRF provides greater long-term pain control |
| -at 9 months, greater pain relief in PRF group than LA group | -No complications reported | ||||
| RFA of lateral C1–C2 joint | |||||
| Halim et al. 2010 [31] | Retrospective study | 86 patients with CeH | Lateral C1–C2 joint PRF using intra-articular anterolateral approach | Roughly 50% of patients had > 50% pain relief at 2 and 6 months and 1 year | PRF of lateral C1–C2 joints is feasible in refractory cases of CeH |
| RFA of C2 DRG | |||||
| Hamer and Purath, 2014 [32] | Retrospective observational | 40 patients with refractory CeH and/or occipital neuralgia | RFN of C2 DRG and/or third occipital nerve | -35% of patients reported complete pain relief | RFN of C2 DRG and/or third occipital nerve can provide > 50% pain relief |
| -70% reported ≥ 80% pain relief | |||||
| Hamer and Purath, 2016 [33] | Retrospective observational | 23 patients with recurrent CeH or occipital neuralgia | Repeat RFA of C2 DRG and/or third occipital nerve | -86.5% of patients reported pain relief lasting for 25.4 weeks | -Repeat RFA is a feasible option for recurrent cases |
| -Repeat RFA showed similar result to first RFA in 59% of patients -32% reported repeat RFA was more effective, while 9% reported first RFA was more effective | -Effectiveness of repeat RFA is the same or better than the first RFA | ||||
| - High likelihood of side effects but well-tolerated | |||||
| Li and Feng, 2019 [34] | Case control | 139 patients with CeH, 87 in the PRF + ESI group and 52 in the ESI only group | PRF for C2 DRG and ESI | -Median pain relief was 4 months for the ESI group and 8 months for the PRF + ESI group | Combination of PRF C2 DRG + ESI is relatively safe, provides sustained pain relief, and improves quality of life |
| -No serious adverse effects reported | |||||
| Lee et al. 2020 [35] | Retrospective observational | Electronic medical records of 45 patients with CeH (initially 114 patients recruited, 45 of which underwent PRF of C2 DRG) | C2 DRG PRF after recurrence of CeH following initial relief 24 hrs after diagnostic C2 DRG block | -40% of patients (18/45, success group) ≥ 50% pain relief after 6 months | C2 DRG PRF effective treatment for CeH, especially for those with positive C2 DRG diagnostic block |
| -No post-procedure complications reported | |||||
| Cryoneurolysis | |||||
| Kvarstein et al. 2019 [36] | RCT, double-blind | 52 patients with unilateral CeH not responding to conservative treatment | After positive diagnostic block, 52 patients were randomly allocated to two groups (3:2): the occipital cryoneurolysis (31) group and the injection group (21) [1 ml depo-medrol (40 mg/ml) + 1 ml bupivacaine (5 mg/ml)] | -Significant reduction in pain > 50% and number of people consuming opioids in both groups | Cryoneurolysis provides substantial but temporary pain relief, and the effect was not significantly different from the injection group |
| -No significant difference seen between groups | |||||
| -Various transient/ minor side effects reported but no significant group differences seen | |||||
CeH: cervicogenic headache, GON: greater occipital nerve, AA: atlantoaxial, RFA: radiofrequency ablation, RFN: radiofrequency neurotomy, PRF: pulsed radiofrequency, ESI: epidural steroid injection, DRG: dorsal root ganglion, NRS: numerical rating scale, VAS: visual analog scale, LA: local anesthetic, US: ultrasound, FL: fluoroscopy, TENS: transcutaneous electrical nerve stimulation.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download