1. Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg. 2011; 112:1392–402.

2. Vincent JL, Rhodes A, Perel A, Martin GS, Della Rocca G, Vallet B, et al. Clinical review: update on hemodynamic monitoring--a consensus of 16. Crit Care. 2011; 15:229.
3. Ameloot K, Palmers PJ, Malbrain ML. The accuracy of noninvasive cardiac output and pressure measurements with finger cuff: a concise review. Curr Opin Crit Care. 2015; 21:232–9.
4. Bubenek-Turconi SI, Craciun M, Miclea I, Perel A. Noninvasive continuous cardiac output by the Nexfin before and after preload-modifying maneuvers: a comparison with intermittent thermodilution cardiac output. Anesth Analg. 2013; 117:366–72.
5. Heusdens JF, Lof S, Pennekamp CW, Specken-Welleweerd JC, de Borst GJ, van Klei WA, et al. Validation of non-invasive arterial pressure monitoring during carotid endarterectomy. Br J Anaesth. 2016; 117:316–23.

6. Fischer MO, Avram R, Cârjaliu I, Massetti M, Gérard JL, Hanouz JL, et al. Non-invasive continuous arterial pressure and cardiac index monitoring with Nexfin after cardiac surgery. Br J Anaesth. 2012; 109:514–21.

7. Critchley LA, Lee A, Ho AM. A critical review of the ability of continuous cardiac output monitors to measure trends in cardiac output. Anesth Analg. 2010; 111:1180–92.

8. Sangkum L, Liu GL, Yu L, Yan H, Kaye AD, Liu H. Minimally invasive or noninvasive cardiac output measurement: an update. J Anesth. 2016; 30:461–80.

9. Westerhof N, Lankhaar JW, Westerhof BE. The arterial Windkessel. Med Biol Eng Comput. 2009; 47:131–41.

10. Broch O, Renner J, Gruenewald M, Meybohm P, Schöttler J, Caliebe A, et al. A comparison of the Nexfin® and transcardiopulmonary thermodilution to estimate cardiac output during coronary artery surgery. Anaesthesia. 2012; 67:377–83.
11. Suzuki T, Suzuki Y, Okuda J, Minoshima R, Misonoo Y, Ueda T, et al. Cardiac output and stroke volume variation measured by the pulse wave transit time method: a comparison with an arterial pressure-based cardiac output system. J Clin Monit Comput. 2019; 33:385–92.

12. Boisson M, Poignard ME, Pontier B, Mimoz O, Debaene B, Frasca D. Cardiac output monitoring with thermodilution pulse-contour analysis vs. non-invasive pulse-contour analysis. Anaesthesia. 2019; 74:735–40.

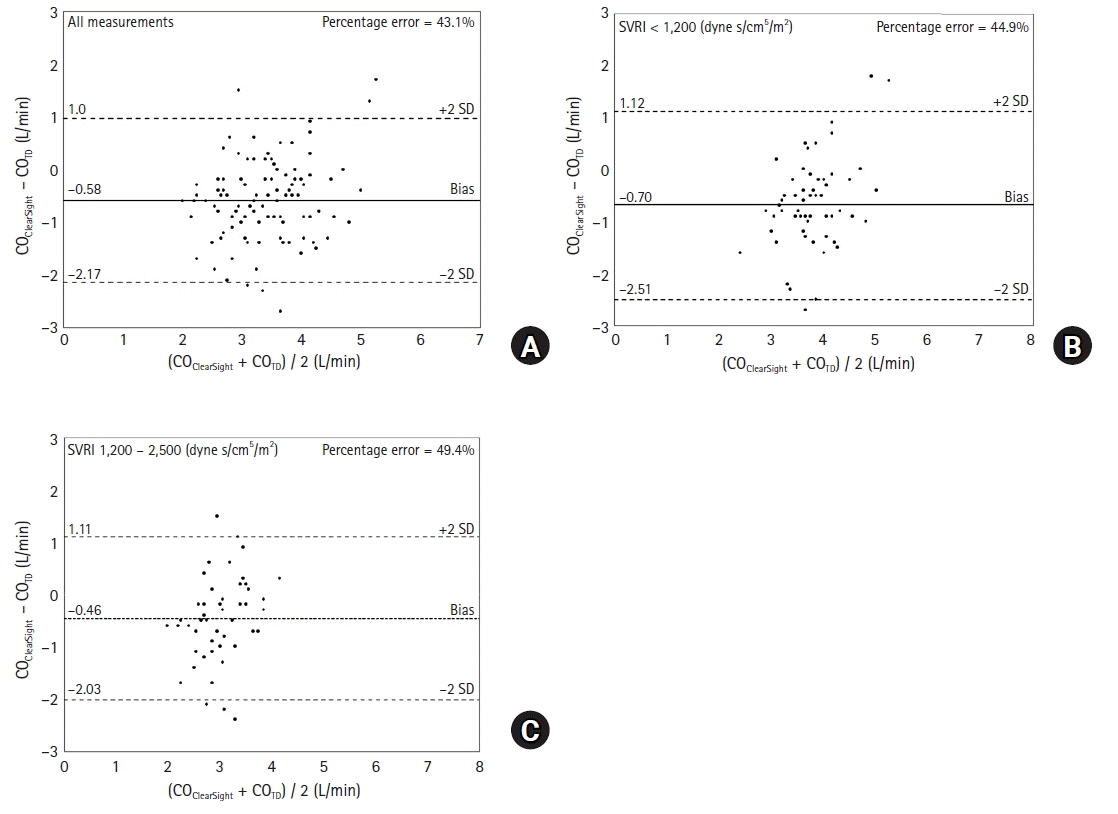
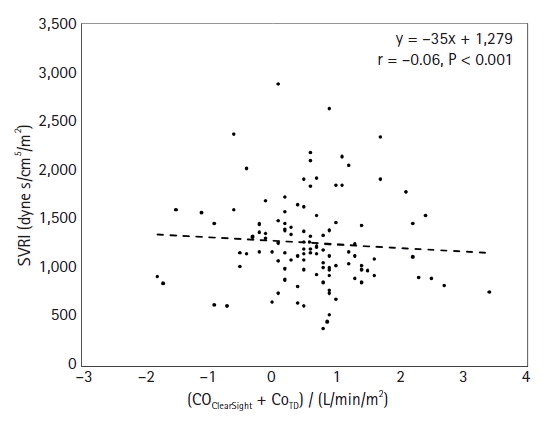
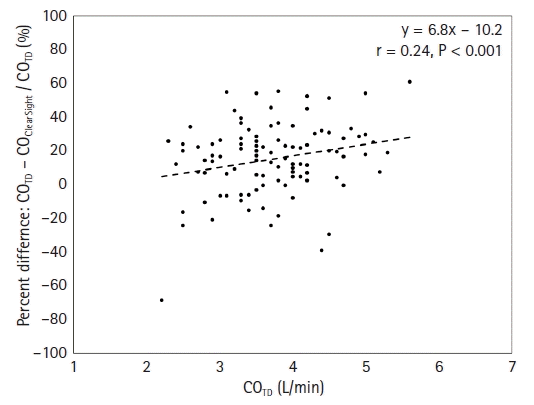
13. Mukai A, Suehiro K, Kimura A, Tanaka K, Yamada T, Mori T, et al. Effect of systemic vascular resistance on the reliability of noninvasive hemodynamic monitoring in cardiac surgery. J Cardiothorac Vasc Anesth. 2021; 35:1782–91.

14. Mantha S, Roizen MF, Fleisher LA, Thisted R, Foss J. Comparing methods of clinical measurement: reporting standards for bland and altman analysis. Anesth Analg. 2000; 90:593–602.

15. Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007; 17:571–82.

16. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986; 1:307–10.

17. Critchley LA, Critchley JA. A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput. 1999; 15:85–91.
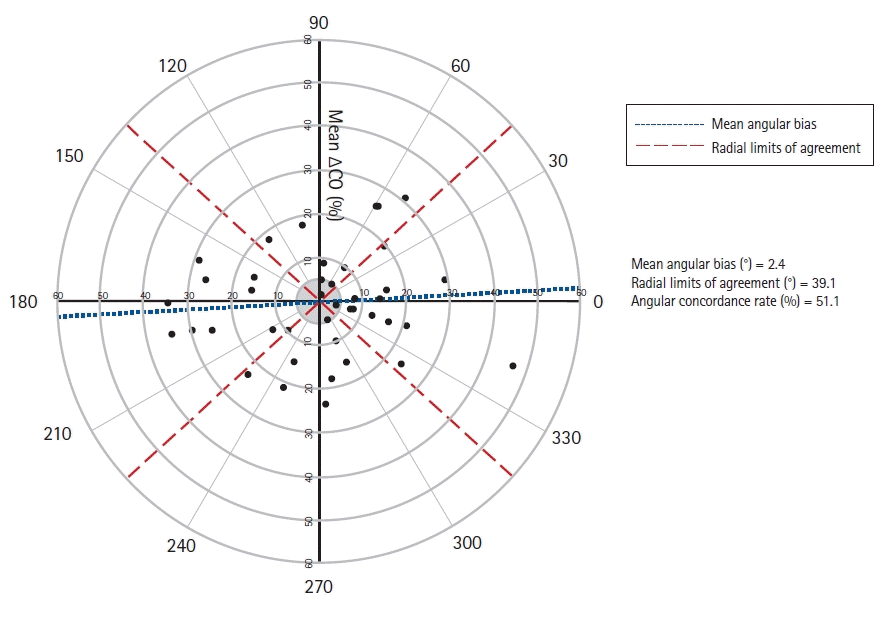
18. Critchley LA, Yang XX, Lee A. Assessment of trending ability of cardiac output monitors by polar plot methodology. J Cardiothorac Vasc Anesth. 2011; 25:536–46.

19. Peyton PJ, Chong SW. Minimally invasive measurement of cardiac output during surgery and critical care: a meta-analysis of accuracy and precision. Anesthesiology. 2010; 113:1220–35.
20. Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013; 48:452–8.

21. Sumiyoshi M, Maeda T, Miyazaki E, Hotta N, Sato H, Hamaguchi E, et al. Accuracy of the ClearSight™ system in patients undergoing abdominal aortic aneurysm surgery. J Anesth. 2019; 33:364–71.

22. Kanazawa H, Maeda T, Miyazaki E, Hotta N, Ito S, Ohnishi Y. Accuracy and trending ability of blood pressure and cardiac index measured by ClearSight system in patients with reduced ejection fraction. J Cardiothorac Vasc Anesth. 2020; 34:3293–9.

23. Grube E, Sinning JM. The "Big Five” complications after transcatheter aortic valve replacement: do we still have to be afraid of them? JACC Cardiovasc Interv. 2019; 12:370–2.
24. Schraverus P, Kuijpers MM, Coumou J, Boly CA, Boer C, van Kralingen S. Level of agreement between cardiac output measurements using Nexfin® and thermodilution in morbidly obese patients undergoing laparoscopic surgery. Anaesthesia. 2016; 71:1449–55.

25. Ostergaard M, Nielsen J, Rasmussen JP, Berthelsen PG. Cardiac output--pulse contour analysis vs. pulmonary artery thermodilution. Acta Anaesthesiol Scand. 2006; 50:1044–9.
26. Bartels SA, Stok WJ, Bezemer R, Boksem RJ, van Goudoever J, Cherpanath TG, et al. Noninvasive cardiac output monitoring during exercise testing: nexfin pulse contour analysis compared to an inert gas rebreathing method and respired gas analysis. J Clin Monit Comput. 2011; 25:315–21.

27. Bogert LW, Wesseling KH, Schraa O, van Lieshout EJ, de Mol BA, van Goudoever J, et al. Pulse contour cardiac output derived from non-invasive arterial pressure in cardiovascular disease. Anaesthesia. 2010; 65:1119–25.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download