Abstract
Background
Catheter-related bladder discomfort (CRBD) is common in patients with a urinary catheter and is a risk factor for emergence agitation (EA). The mainstay of CRBD management is anticholinergics. Dexamethasone inhibits acetylcholine release. This study aimed to evaluate the effects of dexamethasone on postoperative CRBD and EA.
Methods
In this prospective study, 90 patients undergoing urological surgery requiring urinary catheterization were allocated randomly to one of two groups (each n = 45). Before induction of anesthesia, the dexamethasone group received 10 mg (2 ml) of dexamethasone intravenously, while the control group received 2 ml of saline in the same manner. The incidence and severity of CRBD were assessed 0, 1, 2, and 6 h after the patient arrived in the post-anesthesia care unit (PACU) as the primary outcomes. The incidence and severity of EA were also compared during emergence and recovery from anesthesia as secondary outcomes.
Results
The incidences of CRBD in the control group and dexamethasone group at 0, 1, 2, and 6 h postoperatively were 28.9% and 15.6%, 55.6% and 55.6%, 57.8% and 46.7%, and 53.3% and 51.1%, respectively. The incidence and severity of CRBD assessed at 0, 1, 2, and 6 h postoperatively did not show intergroup differences. The incidence and severity of EA in the operating room and PACU also showed no difference between the groups.
Go to : 
Urinary catheterization is performed during urological surgery to empty and irrigate the bladder, prevent urethral injury, the formation of blood clots, reimplantation of cancer cells, and short-term voiding failure, and to allow monitoring of postoperative bleeding and urinary output. However, 47–90% of patients with a urinary catheter experience catheter-related bladder discomfort (CRBD), defined as an unpleasant and burning sensation in the urethra and suprapubic area [1]. CRBD causes significant postoperative distress, often accompanied by behavioral responses, such as loud complaining, flailing limbs, and attempts to remove the urinary catheter [2,3]. These responses can lead to increased medical-staff workload, exacerbation of postoperative pain, poor quality of life, extended hospital stay, and an increased incidence of postoperative complications, including bleeding, surgical wound dehiscence, arrhythmias, and hemodynamic instability [2]. Therefore, prevention and early management of CRBD is clinically important, and many interventions have been proposed [1–4]. The mainstay of treatment for CRBD is anticholinergic agents that inhibit M2, and particularly M3, muscarinic receptors [1,2]. Inhibitors of prostaglandin (PG) synthesis, such as paracetamol, also reduce the severity of CRBD [3].
Meanwhile, the presence of a urinary catheter, as well as CRBD secondary to an indwelling urinary catheter, can increase the risk for emergence agitation (EA) [5,6]. EA may result in an increase in bleeding at the surgical site, injury to the self or medical staff, unintended extubation, accidental removal of catheters, and increased medical care costs [6].
The synthetic glucocorticoid dexamethasone inhibits acetylcholine release by increasing inhibitory M2 muscarinic receptor function [7], and represses PG synthesis by antagonizing upregulation of cyclooxygenase (COX) [8]. In addition, the anti-inflammatory, antiemetic, and analgesic effects of dexamethasone may reduce sore throat, postoperative nausea and vomiting (PONV), and pain, which are known risk factors for EA [6,9,10]. This study was designed to evaluate the effects of dexamethasone on CRBD and EA in patients undergoing elective urological surgery.
Go to : 
The Institutional Review Board of Konyang University Hosptal approved this investigation (approval number: KYUH 2018-02-001-002) and the protocol was registered with the Korean Clinical Research Information Service. This prospective, randomized, placebo-controlled study was conducted from May 2018 through October 2018 in a single university hospital (Konyang University Hostital, Daejeon, Korea) after obtaining written informed consent from all participants. This clinical research was done following the ethical principles for medical research involving human subjects in accordance with the Helsinki Declaration 2013. The study included 19 to 80-year-old patients with American Society of Anesthesiologists physical status I–III, who were scheduled for urological surgery requiring urinary catheterization. A patient was excluded if any of the following criteria were met: (1) immunosuppression; (2) active infection; (3) bladder outlet obstruction; (4) overactive bladder (OAB; urinary frequency > 3 times per night or > 8 times in 24 h); (5) prostate hypertrophy; (6) end-stage renal disease; (7) neuropsychological disorder or cognitive impairment; (8) uncontrolled diabetes; or (9) undergoing urological surgery with an anesthesia duration expected to exceed 6 h (considering the plasma half-life of dexamethasone of approximately 6 h) [11].
Patients were allocated randomly (1 : 1 allocation ratio) to one of two groups (control or dexamethasone group) using a random number table generated using online randomization software (www.randomizer.org). The allocations were concealed in opaque envelopes and opened when the patient arrived in the preanesthetic holding area by the anesthetic nurse responsible for preparing the study drugs (saline for the control group and dexamethasone for the dexamethasone group). The anesthetic nurse was blinded to the purpose of the study and was not involved in data collection. In addition, the patients, the anesthesiologist who evaluated the outcome variables, and the urologist who performed the urinary catheterization were blinded to group allocation.
All patients fasted for at least 8 h and entered the operating room without premedication. Routine monitoring included pulse oximetry, electrocardiography, noninvasive automated blood pressure monitoring, the Patient State Index (PSI) (SedLine®; Masimo Corp., USA), and neuromuscular train-of-four (TOF) acceleromyography (TOF-Watch SX®; Organon Ltd., Ireland) on the adductor pollicis muscle. Immediately before induction of anesthesia, patients in the dexamethasone group received 10 mg (2 ml; 5 mg/ml) of dexamethasone sodium phosphate (Yuhan Co., Korea) intravenously, while patients in the control group received 2 ml of normal saline intravenously. Anesthesia was induced via intravenous injection of propofol (1.5–2 mg/kg) and fentanyl (1–2 μg/kg). Orotracheal intubation was facilitated with an intravenous injection of rocuronium (0.6 mg/kg). Volume-controlled mechanical ventilation was initiated at a tidal volume of 8 ml/kg, respiratory rate of 12 breaths/min, and positive end-expiratory pressure of 5 cmH2O; the target end-tidal carbon dioxide concentration of 30–40 mmHg was maintained by adjusting the respiratory rate. Anesthesia was maintained with oxygen and nitrous oxide (O2:N2O; 50:50) and desflurane (3–8 vol% end-tidal concentration) to keep the PSI at 25–50. At the end of surgery, a urinary catheter was placed by a urologist after lubrication with 2% lidocaine jelly (Instillagel®; Farco-Pharma GmbH, Germany). The size of the urethral catheter and volume of the balloon were at the discretion of the urologist. The balloons were filled with normal saline. At the end of surgery, and after confirming a TOF count of at least 2, all inhalation anesthetics were stopped and the neuromuscular block was reversed with 40–50 μg/kg neostigmine and 10 μg/kg glycopyrrolate. After confirming recovery of the neuromuscular block (TOF ratio ≥ 0.9), spontaneous respiration (respiratory rate > 12/min; tidal volume > 5 mg/kg), and PSI > 75, extubation was performed by an anesthesiologist. The time between stopping the inhalation anesthetics and extubation was measured. EA was assessed after stopping the inhalation anesthetics until 5 min after extubation in the operating room, and between admission and discharge from the post-anesthesia care unit (PACU) using the Riker Sedation-Agitation Scale (RSAS; 1 = unarousable, 2 = very sedated, 3 = sedated, 4 = calm and cooperative, 5 = agitated and calm to verbal instructions, 6 = very agitated, requiring restraint, and 7 = pulling at the tracheal tube, trying to remove catheters, or striking the staff) [12]. The highest RSAS scores in the operating room and PACU were recorded; scores ≥ 5 was considered indicative of the presence of EA.
All patients were observed for at least 1 h in the PACU. Postoperative pain was rated using a numerical rating scale (NRS; 0 = no pain; 10 = worst pain imaginable). The incidence and severity of CRBD were assessed at 0, 1, 2, and 6 h after patient arrival in the PACU by an anesthesiology resident using a 4-point scale (none = patient did not complain of any CRBD symptoms even when asked, such as urge to urinate or discomfort in the suprapubic region; mild = complaint of CRBD symptoms only on direct questioning; moderate = spontaneous complaint of CRBD symptoms, without any behavioral responses, such as attempts to remove the urethral catheter, flailing limbs, loud complaints; severe = spontaneous complaint of CRBD symptoms with a behavioral response) [13–16]. The mild, moderate, and severe categories were all considered indicative of CRBD. If the patient required an analgesic due to postoperative pain or CRBD, 25–50 mg pethidine was administered intravenously. The RSAS was evaluated at the same time as CRBD. An RSAS score ≤ 3 was recorded as sedation. Adverse events during the first 6 h postoperatively were recorded and analyzed.
The primary endpoints were the incidence and severity of CRBD 0, 1, 2, and 6 h after the patient arrived in the PACU. The secondary endpoints were the incidence and severity of EA during emergence and recovery from anesthesia.
Previous studies have reported an incidence of CRBD between 65% and 80% at 0 h postoperatively, following reversal of neuromuscular blockade with glycopyrrolate and neostigmine [13,17]. Based on these studies, we assumed that the incidence rates of postoperative CRBD would be around 65% in the control group. With an effect size (h) of 0.662, a power of 0.8, α-value of 0.05 (two-sided), and allocation ratio of 1 : 1, a sample size of 42 patients per group was required to detect a 50% reduction in the incidence of CRBD after administering dexamethasone. Considering a 5% dropout rate, 45 patients were enrolled in each group.
Statistical analyses were performed using SPSS StatisticsTM software (ver. 18.0 for Windows; IBM SPSS Inc., USA). The distribution of continuous variables was assessed with the Kolmogorov–Smirnov test; normally distributed variables were analyzed using Student’s t-test and non-normally distributed variables were analyzed using the Mann–Whitney U test. Categorical variables were analyzed using the χ2 test, the χ2 test for trends (linear-by-linear association), or Fisher’s exact, as appropriate. A P value < 0.05 was considered significant. Cohen’s effect sizes d and h were also used to compare continuous and categorical variables, respectively.
Go to : 
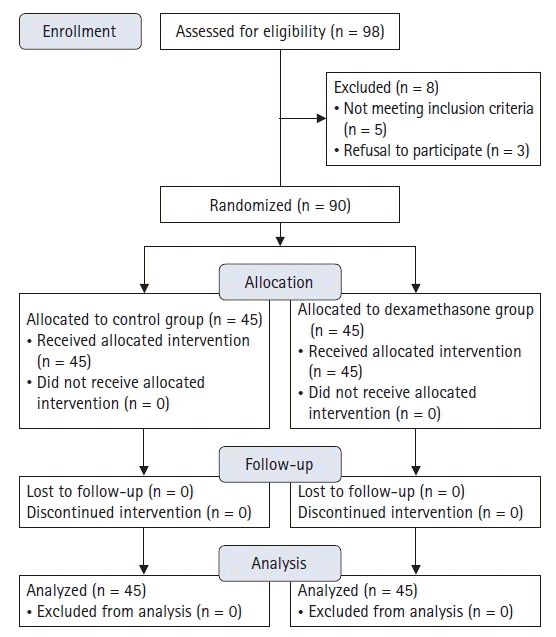
A total of 98 patients were screened for inclusion in the study. Eight patients were excluded due to prostate hypertrophy (n = 3), OAB (n = 1), neuropsychological disorder (n = 1), or refusal to participate (n = 3). Thus, 90 patients were randomly allocated to one of the two groups and completed the study (Fig. 1). There were no differences in the demographic and operative data between the two groups (Table 1).
Demographic and Operative Data
The incidence rates of CRBD in the control and dexamethasone groups were 28.9% and 15.6% at 0 h, 55.6% and 55.6% at 1 h, 57.8% and 46.7% at 2 h, and 53.3% and 51.1% at 6 h following PACU admission, but the differences between the two groups were not significant (P = 0.128, 1.000, 0.291, and 0.833, respectively). There were no differences in the severity of CRBD, assessed at 0, 1, 2, and 6 h after the patient arrived in the PACU, between the groups (P = 0.057, 0.357, 0.086, and 0.739, respectively) (Table 2).
Incidence and Severity of Postoperative CRBD
The recovery and postoperative data are presented in Table 3. The incidence rates of EA in the control and dexamethasone groups were 15.6% and 6.7% in the operating room (P = 0.315), and 24.4% and 15.6% in PACU (P = 0.292), respectively. The severity of EA was not different between the two groups in the operating room or PACU (P = 0.157 and 0.228 in the operating room and PACU, respectively). In addition, time to extubation, the postoperative NRS pain score, and the requirement for analgesics were comparable between the groups (all P > 0.05).
Recovery and Postoperative Data
Adverse events during 6 h postoperatively, including sore throat, hoarseness, dry mouth, nausea, vomiting, headache, dizziness, dyspnea, and sedation, were not significantly different between the groups (Table 4).
Adverse Events
Go to : 
This study assessed the effects of dexamethasone on CRBD and EA in patients who received a urinary catheter after urological surgery. Intravenous administration of dexamethasone (10 mg) before induction of anesthesia did not reduce the incidence or severity of CRBD at 0, 1, 2, and 6 h after urological surgery. In addition, dexamethasone did not affect the incidence or severity of EA evaluated in the operating room and PACU after surgery.
The clinical symptoms (discomfort in the suprapubic region, burning sensation in the urethra, urinary frequency, and urgency) of CRBD mimic those of OAB (urinary urgency with or without urge incontinence) [15]. In addition, the pathophysiology of CRBD and OAB is associated with contraction of the involuntary detrusor muscle [3]. In CRBD, the urinary catheter stimulates the afferent nerve of the bladder, leading to acetylcholine release and muscarinic receptor-mediated involuntary contraction of the detrusor smooth muscle of the bladder [18]; the detrusor muscle of the bladder contains several subtypes (M1–M5) of the muscarinic receptors, but the M2 and M3 receptors are predominant. The M2 and M3 receptors are present in a 3 : 1 ratio. A minority of M3 muscarinic receptors are primarily responsible for contracting the detrusor, whereas M2 muscarinic receptors contribute to indirect contractions and/or inhibit detrusor relaxation [19]. Obstruction of the urinary tract, injuries to the bladder mucosa, nerve stimulation, contraction of the detrusor muscle, and inflammatory mediators promote PG synthesis [16,20]. Inflammatory stimulation due to urinary bladder catheterization leads to an increase in PGE2 synthesis due to increased activation of COX-2, which may lead to the clinical symptoms of CRBD [3]. Therefore, antimuscarinic agents (e.g., oxybutynin, butylscopolamine, tolterodine, and glycopyrrolate), analgesics with anticholinergic activity (e.g., nefopam, tramadol, dexmedetomidine, and ketamine), and COX inhibitors (paracetamol and ketorolac), which inhibit PG synthesis, have been studied and can reduce CRBD [2,16].
Dexamethasone, a drug that is commonly used during the perioperative period, decreased the release of acetylcholine in an in vivo study of airway parasympathetic neurons, by increasing mRNA expression of the M2 receptor. Simultaneously, dexamethasone enhances the degradation of acetylcholine by increasing cholinesterase activity [7]. Dexamethasone also reduces the COX-2 transcription rate and inhibits COX activity and the release of COX-2 and PGE2 [8]. Therefore, we expected dexamethasone to help reduce CRBD, but this was not observed. The reasons for the differences in our results and expectations are uncertain, but a few possible explanations are as follows. First, dexamethasone, a synthetic glucocorticoid, increases the glomerular filtration rate in both experimental animals and humans [21]. In an experimental study [22], dexamethasone significantly increased the urine output without activating the renin-angiotensin-aldosterone system. We speculate that the increase in urine output due to the diuretic effect of dexamethasone may have contributed to the induction of symptoms of CRBD, such as urinary urgency. Second, we used neostigmine and glycopyrrolate (10 μg/kg) to reverse the neuromuscular block caused by rocuronium. Glycopyrrolate is an anticholinergic agent with high affinity for the M3 receptor [23]. Injecting 0.3 mg glycopyrrolate before inducing anesthesia decreases the incidence and severity of CRBD in the PACU compared to saline [14]. In addition, 10 μg/kg glycopyrrolate administered after surgery decreases the early postoperative incidence and severity of CRBD compared to the anticholinergic agent atropine [13]. Compared to these studies [13,14], the dosage of glycopyrrolate in our study was larger or the same. In addition, the incidence of CRBD at 0 h and 1 h postoperatively in the control group (28.9% and 55.6%, respectively) was lower or comparable to that in the glycopyrrolate groups in a previous study (65% and 54%, respectively) [13]. Therefore, reducing the incidence and severity of CRBD in the control group after glycopyrrolate administration may have decreased the difference between the two groups in this study.
Sex (male), the diameter of the urinary catheter (≥ 18 Fr), and type of surgery (urological surgery, especially endourology and bladder resection) are independent risk factors for CRBD [2]. CRBD exacerbates postoperative pain and may increase the incidence of EA [1,2]. Risk factors of EA include male sex, obesity, pre-existing mental health problems, type of surgery (oral cavity surgery or otolaryngological surgery), longer duration of surgery, emergency operation, the presence of invasive devices (e.g., urinary catheter or endotracheal tube), CRBD, voiding urgency, postoperative pain, PONV, sore throat, history of substance dependence, and method of anesthesia (inhalation anesthesia) [5,6,10]. Although the pathophysiological mechanism of EA remains unknown, the mainstay of EA management is prevention by eliminating risk factors [5,6].
We administered fentanyl (1–2 μg/kg) during the induction of anesthesia to prevent hemodynamic instability due to tracheal intubation. The analgesic dosage of fentanyl is 1–1.5 μg/kg [24]. Fentanyl has a prophylactic effect, preventing sevoflurane- and desflurane-related EA [25]. CRBD is resistant to conventional analgesics, such as opioids [16]; thus, fentanyl may not affect CRBD. However, considering the pharmacokinetic properties of fentanyl (duration of action of 30–60 min, elimination half-life of 219 min) [24] and the duration of surgery in this study, fentanyl would have reduced EA of the control group so that there was no difference between the two groups in this study. It is also possible that there was no difference in EA between the two groups because dexamethasone did not reduce CRBD, postoperative pain, PONV, or sore throat, which are risk factors for EA.
This study had several limitations. First, as mentioned previously, glycopyrrolate used in this study may have affected the incidence and severity of CRBD. If anticholinergics such as glycopyrrolate were not used for reversal of a rocuronium-induced neuromuscular blockade using sugammadex, the effects of dexamethasone on CRBD could be more clearly evaluated. Considering the incidence of CRBD (28.9% and 15.6% in the control and dexamethasone groups, respectively) at arrival in the PACU and the effects of glycopyrrolate on CRBD, additional studies with larger sample sizes and different types of reversal drugs (e.g., sugammadex) are required. Second, the clinical effects of dexamethasone are associated with the dose administered [26]. Therefore, the effect of dexamethasone at a dose > 10 mg on CRBD and EA is unknown, and further research is needed. Third, although there was no statistical difference, the type of surgery, sex, and diameter of the urinary catheter, which are risk factors for CRBD, were not the same in both groups, which may have affected the incidence and severity of CRBD. In addition, sex, type of surgery, sore throat, PONV, and postoperative pain are risk factors for EA and may have affected the incidence and severity of EA. Future studies controlling these factors are needed. Finally, as the sample size was calculated based on the incidence of CRBD, the secondary outcome variables, i.e., the incidences of EA in the operating room and PACU, had lower statistical power values of 0.27 and 0.18, respectively, and it is possible that a type II error occurred. Therefore, further research with greater numbers of subjects is necessary to confirm the statistical significance of the differences in EA between the groups.
In conclusion, intravenous administration of 10 mg dexamethasone before induction of anesthesia did not further decrease the incidence or severity of either CRBD or EA in patients undergoing urological surgery.
Go to : 
Notes
Author Contributions
Sung-Ae Cho (Formal analysis; Writing – original draft; Writing – review & editing)
Inho Huh (Data curation; Investigation)
Seok-Jin Lee (Data curation; Investigation)
Tae-Yun Sung (Conceptualization; Formal analysis; Methodology; Supervision; Writing – original draft; Writing – review & editing)
Gwan Woo Ku (Conceptualization; Formal analysis)
Choon-Kyu Cho (Data curation; Investigation)
Young Seok Jee (Data curation; Investigation)
Go to : 
References
1. Li S, Song L, Ma Y, Lin X. Tramadol for the treatment of catheter-related bladder discomfort: a randomized controlled trial. BMC Anesthesiol. 2018; 18:194.

2. Bai Y, Wang X, Li X, Pu C, Yuan H, Tang Y, et al. Management of catheter-related bladder discomfort in patients who underwent elective surgery. J Endourol. 2015; 29:640–9.

3. Ergenoglu P, Akin S, Yalcin Cok O, Eker E, Kuzgunbay B, Turunc T, et al. Effect of intraoperative paracetamol on catheter-related bladder discomfort: a prospective, randomized, double-blind study. Curr Ther Res Clin Exp. 2012; 73:186–94.

4. Hur M, Park SK, Yoon HK, Yoo S, Lee HC, Kim WH, et al. Comparative effectiveness of interventions for managing postoperative catheter-related bladder discomfort: a systematic review and network meta-analysis. J Anesth. 2019; 33:197–208.

5. Yu D, Chai W, Sun X, Yao L. Emergence agitation in adults: risk factors in 2,000 patients. Can J Anaesth. 2010; 57:843–8.

6. Lee SJ, Sung TY. Emergence agitation: current knowledge and unresolved questions. Korean J Anesthesiol. 2020; 73:471–85.

7. Jacoby DB, Yost BL, Kumaravel B, Chan-Li Y, Xiao HQ, Kawashima K, et al. Glucocorticoid treatment increases inhibitory m(2) muscarinic receptor expression and function in the airways. Am J Respir Cell Mol Biol. 2001; 24:485–91.

8. Newton R, Seybold J, Kuitert LM, Bergmann M, Barnes PJ. Repression of cyclooxygenase-2 and prostaglandin E2 release by dexamethasone occurs by transcriptional and post-transcriptional mechanisms involving loss of polyadenylated mRNA. J Biol Chem. 1998; 273:32312–21.
9. Thomas S, Beevi S. Dexamethasone reduces the severity of postoperative sore throat. Can J Anaesth. 2007; 54:897–901.

10. Sajedi P, Baghery K, Hagibabie E, Mehr AM. Prophylactic use of oral acetaminophen or IV dexamethasone and combination of them on prevention emergence agitation in pediatric after adenotonsillectomy. Int J Prev Med. 2014; 5:721–7.
11. O’Sullivan BT, Cutler DJ, Hunt GE, Walters C, Johnson GF, Caterson ID. Pharmacokinetics of dexamethasone and its relationship to dexamethasone suppression test outcome in depressed patients and healthy control subjects. Biol Psychiatry. 1997; 41:574–84.

12. Riker RR, Picard JT, Fraser GL. Prospective evaluation of the sedation-agitation scale for adult critically ill patients. Crit Care Med. 1999; 27:1325–9.

13. Kim HC, Lim SM, Seo H, Park HP. Effect of glycopyrrolate versus atropine coadministered with neostigmine for reversal of rocuronium on postoperative catheter-related bladder discomfort in patients undergoing transurethral resection of bladder tumor: a prospective randomized study. J Anesth. 2015; 29:831–5.

14. Kim JA, Min JH, Lee HS, Jo HR, Je UJ, Paek JH. Effects of glycopyrrolate premedication on preventing postoperative catheter-related bladder discomfort in patients receiving ureteroscopic removal of ureter stone. Korean J Anesthesiol. 2016; 69:563–7.

15. Cheon YW, Kim SH, Paek JH, Kim JA, Lee YK, Min JH, et al. Effects of nefopam on catheter-related bladder discomfort in patients undergoing ureteroscopic litholapaxy. Korean J Anesthesiol. 2018; 71:201–6.

16. Park JY, Hong JH, Yu J, Kim DH, Koh GH, Lee SA, et al. Effect of ketorolac on the prevention of postoperative catheter-related bladder discomfort in patients undergoing robot-assisted laparoscopic radical prostatectomy: a randomized, double-blinded, placebo-controlled study. J Clin Med. 2019; 8:759.

17. Agarwal A, Dhiraaj S, Pawar S, Kapoor R, Gupta D, Singh PK. An evaluation of the efficacy of gabapentin for prevention of catheter-related bladder discomfort: a prospective, randomized, placebo-controlled, double-blind study. Anesth Analg. 2007; 105:1454–7.

18. Kwon Y, Jang JS, Hwang SM, Lee JJ, Tark H. Intraoperative administration of dexmedetomidine reduced the postoperative catheter-related bladder discomfort and pain in patients undergoing lumbar microdiscectomy. J Anesth. 2018; 32:41–7.

19. Abrams P, Andersson KE, Buccafusco JJ, Chapple C, de Groat WC, Fryer AD, et al. Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol. 2006; 148:565–78.

20. Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev. 2004; 84:935–86.

21. Baylis C, Handa RK, Sorkin M. Glucocorticoids and control of glomerular filtration rate. Semin Nephrol. 1990; 10:320–9.
22. Liu C, Ge N, Zhai JL, Zhang JX. Dexamethasone-induced diuresis is associated with inhibition of the renin-angiotensin-aldosterone system in rats. Kaohsiung J Med Sci. 2016; 32:614–9.

23. Haddad EB, Patel H, Keeling JE, Yacoub MH, Barnes PJ, Belvisi MG. Pharmacological characterization of the muscarinic receptor antagonist, glycopyrrolate, in human and guinea-pig airways. Br J Pharmacol. 1999; 127:413–20.

24. Vahedi HS, Hajebi H, Vahidi E, Nejati A, Saeedi M. Comparison between intravenous morphine versus fentanyl in acute pain relief in drug abusers with acute limb traumatic injury. World J Emerg Med. 2019; 10:27–32.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download