Introduction
The rate of perioperative hypothermia, defined as a reduction in body temperature to < 36.0°C during the perioperative period, is typically 50–90%, even during short and simple surgeries [
1,
2]. Managing perioperative body temperature is very important because even mild hypothermia can cause complications, including cardiac morbidity, poor drug metabolism, delayed recovery from anesthesia, greater blood loss in association with platelet dysfunction and coagulopathy, delayed wound recovery, and greater frequency of surgical site infections [
3]. A forced-air warmer is the most commonly used device to prevent perioperative hypothermia and provides warmth not only by transferring convective heat to the body but also by preventing heat loss from the covered area [
4–
6].
Patients undergoing spine surgery in the prone position tend to be susceptible to hypothermia because of the long surgical duration and large exposed skin surface area associated with the procedure [
6]. Perioperative hypothermia is also associated with ophthalmic complications during spine surgery in the prone position, so preventing hypothermia is important [
7]. The effectiveness of forced-air warming underbody blankets has been reported, but they are more expensive than conventional warming devices and thus not particularly popular [
8]. Therefore, forced-air warming over-body blanket for the upper or lower body is often used, depending on the location of the surgical site.
Previous studies have reported that lower-body warming is more effective in the supine position because of the larger body surface area (BSA) covered [
9,
10]. However, another study reported that, in the lateral decubitus position, upper-body warming was more effective than lower-body warming due to the padding between the legs in the latter case [
11]. According to a preliminary retrospective study conducted at our institution, upper-body warming results in a significantly lower incidence of hypothermia compared with lower-body warming, during spine surgery in the prone position (unpublished data). However, few studies have compared the effectiveness of upper- and lower-body warming during spine surgery in the prone position [
12]. We hypothesized that an upper-body blanket would be superior to a lower-body blanket to prevent perioperative hypothermia in patients undergoing spine surgery in the prone position. Thus, we compared the warming effects of upper- and lower-body forced-air blankets in patients undergoing spine surgery in the prone position.
Go to :

Materials and Methods
This prospective randomized controlled trial was approved by the institutional ethics committee (SCHUH 2018-12-009-001) of Soonchunhyang university hospital in Seoul and registered at the Clinical Research Information Service (CRIS) clinical trials registry (KCT0003728). The trial was performed from March 2019 to August 2019. All patients were given information about the trial, and all provided written informed consent. This manuscript adheres to the relevant CONSORT guidelines. The research was performed following the ethical principles for medical research involving human subjects of the 1964 Declaration of Helsinki and all of its subsequent revisions (revised 2013).
Study participants and inclusion/exclusion criteria
The trial included 120 patients (aged ≥ 19 years) with an American Society of Anesthesiologists physical status (ASA-PS) of I–III who were scheduled to undergo elective spine surgery in the prone position. Exclusion criteria were body mass index (BMI) > 35 kg/m2, preoperative body temperature > 38°C or < 36°C, and pregnancy.
Randomization and masking
Using Excel software (2016; Microsoft Corp., USA), patients were randomly allocated to the upper- or lower-warming groups (both n = 60) by a computer-generated blocked randomization scheme (block size 4, 6; 1 : 1 allocation ratio). The anesthesiologists (who anesthetized the patients and supervised the warming) were not blinded to the group allocation, whereas the patients and study nurse (who collected the pre- and post-operative data) were blinded.
General procedures
After arrival in the operating room, all patients were fully covered with a cotton blanket. Standardized monitoring and anesthesia induction (using 1–2 mg/kg 1% propofol and 0.6 mg/kg rocuronium) were performed. Catheters were inserted into the urethra, radial artery, or internal jugular vein as needed, with minimal exposure of the skin to ambient air. Then, the patient was placed in the prone position. Standardized anesthesia was maintained using desflurane and remifentanil.
After prone positioning, the specified upper and lower blankets were placed over the back and both arms in the upper-warming group, and over the lower buttocks and both legs in the lower-warming group by the anesthesiologist. As most surgical fields for spine surgery are from the T4 dermatome to the coccyx, a Warm TouchTM upper-body blanket (Medtronic, Ireland) was taped above the T4 spinous process level, and covered the upper extremities and upper trunk above the T4 dermatome. A Warm TouchTM lower-body blanket (Medtronic) was taped below the coccyx and covered both legs and the lower buttocks below the coccyx. The entire body of all patients was covered with a surgical drape, except for the surgical field and head. After surgical draping, intraoperative warming using a forced-air warmer (WarmTouch™ WT 6000 Warming Unit; Medtronic) was applied until the end of the surgery. The temperature was adjusted to 45°C when the core body temperature was < 36.5°C, and to 40°C when the core body temperature was 36.5–37.5°C. The warmer was turned off when the core body temperature was > 37.5°C. A breathing circuit that allows for intraoperative heating/humidification was used in all patients; no other warming devices were used.
At the end of surgery, we removed the forced-air blanket, placed the patient in the supine position, and warmed the whole body with a cotton blanket during emergence from anesthesia. The patient’s consciousness and spontaneous respiration were restored, and the nasopharyngeal thermometer and tracheal tube were removed. The patients were transferred to the post-anesthesia care unit (PACU). If tympanic temperature measurement and the postoperative evaluations indicated hypothermia, warming was actively performed using the forced-air warmer as described above.
Measurements
The baseline patient characteristics were recorded preoperatively, including age, sex, weight, height, BMI, ASA-PS, and level of spine surgery. BSA was calculated using the Dubois formula.
To evaluate the primary endpoint of the incidence of perioperative hypothermia, tympanic temperature was measured using a Thermoscan®, an infrared tympanic thermometer (IRT 4020; Braun, USA), by a masked nurse in the pre-anesthetic holding area, and in the PACU every 10 min (up to 30 min) after arrival in the PACU [
13]. The right and left tympanic temperatures were measured, and the average value was calculated. The nasopharyngeal temperature was measured using a thermometer (ETP1040; Ewha Biomedics, Korea) at a depth of 9–10 cm in the nasopharynx immediately after induction of anesthesia [
14]. Readings were obtained every 15 min till the end of surgery.
The secondary endpoints were perioperative temperature changes, postoperative thermal comfort (100-mm visual analogue scale: 0 mm = coldest imaginable, 50 mm = pleasant, 100 mm = warmest imaginable) and shivering (0 = no shivering; 1 = intermittent, low intensity; 2 = moderate shivering; 3 = continuous intense shivering) scores, patient satisfaction regarding temperature management (0 = very dissatisfied, 1 = dissatisfied, 2 = neutral, 3 = satisfied, 4 = very satisfied), and PACU duration. All patients were trained in the use of the thermal comfort scale in the ward on the day before surgery. A masked nurse asked the patient to complete the thermal comfort and shivering scales every 10 min following arrival in the PACU (up to 30 min). Before leaving the PACU, the patients were asked to rate the satisfactoriness of the perioperative temperature on a five-point Likert scale. The length of stay in the PACU and any adverse effects of forced-air warming were also recorded.
The ambient temperatures in the operating room and PACU were recorded on arrival and discharge, and the average temperature was calculated. The durations of the ‘unwarmed’ (from arrival in the operating room to the start of intraoperative warming) and anesthetic periods were recorded. The intraoperative fluid volume, blood loss, and transfusion requirement were also recorded by the anesthesiologist.
Sample size and statistical analyses
Min et al. [
11] reported that the incidence of intraoperative hypothermia in the lateral decubitus position during thoracoscopic surgery was 33.87% in their upper-warming group and 57.38% in their lower-warming group. Assuming that the incidence of intraoperative hypothermia would be reduced by a similar degree in our study, we calculated that 60 patients per group were required with an α of 0.05 for a one-tailed test, power of 80%, and a drop-out rate of 10%.
Statistical analyses were performed using SPSS for Windows software (version 26.0; IBM Corp., USA). The two groups were compared using Student’s t-test or the Mann–Whitney rank-sum test for continuous data, after checking for normality with the Shapiro–Wilk test, and by the chi-square or Fisher’s exact test for categorical data. All analyses in this trial were conducted in an intention-to-treat manner because dropout data were missing.
Perioperative body temperature data were plotted and analyzed using a mixed-effects model with a first-order autoregressive covariance structure. The fixed effects in the mixed-effects model included group, time, and the interaction between group and time. Subjects were included as a random effect. Post-hoc testing using Bonferroni’s method for pairwise group comparisons was performed when the results of the mixed-effects model were significant.
Multivariable logistic regression analysis was performed post-hoc to identify variables affecting intraoperative hypothermia (< 36.0°C) and severe intraoperative hypothermia (< 35.0°C). Variation inflation factors of BMI, BSA, weight, height, and the BSA/weight ratio were more than 10.0, which caused multicollinearity in the multiple regression. Therefore, the BSA/weight ratio was selected as the morphometric variable. In total, 11 variables were included (BSA/weight × 1,000, group, sex, age, ASA-PS, anesthetic duration, surgery type [> 2 levels], unwarmed duration (from arrival in the operating room to the start of intraoperative warming), operating room ambient temperature, preoperative body temperature, and fluid administration [> 1,000 ml]) as independent variables in the multivariable logistic regression. The backward stepwise elimination method was used to select the variables based on the log-likelihood ratio.
Continuous data are presented as mean and standard deviation (SD) or median and 25
th and 75
th percentiles, and categorical data as frequencies with percentages. A P value < 0.05 was considered significant, and a temperature difference between the intervention and control groups of 0.2°C was defined as significant based on the National Institutes for Health and Clinical Excellence guidelines [
15].
Go to :

Results
In total, 126 patients were screened. Three patients were excluded because they met the exclusion criteria and another three refused to participate. Thus, 120 patients were ultimately enrolled in the study and were divided randomly into the upper- and lower-warming groups (both n = 60). Two patients in the upper-warming group dropped out just before surgery (one due to hyperthermia [38.4°C] and one due to canceled surgery). Continuous intraoperative warming was stopped in two patients in the lower-warming group (due to a machine error in one patient and failure to record intraoperative core temperature data due to a thermometer module error in another patient). Therefore, data from 116 patients were analyzed (58 patients in each group). Two patients in the upper-warming group and one in the lower-warming group were transferred to the intensive care unit for close postoperative observation by the surgeon; thus, postoperative data from these three patients could not be obtained. Moreover, thermal comfort and satisfaction-scale data could not be obtained in two patients (one each in the upper- and lower-warming groups) who showed postoperative delirium in the PACU (
Fig. 1).
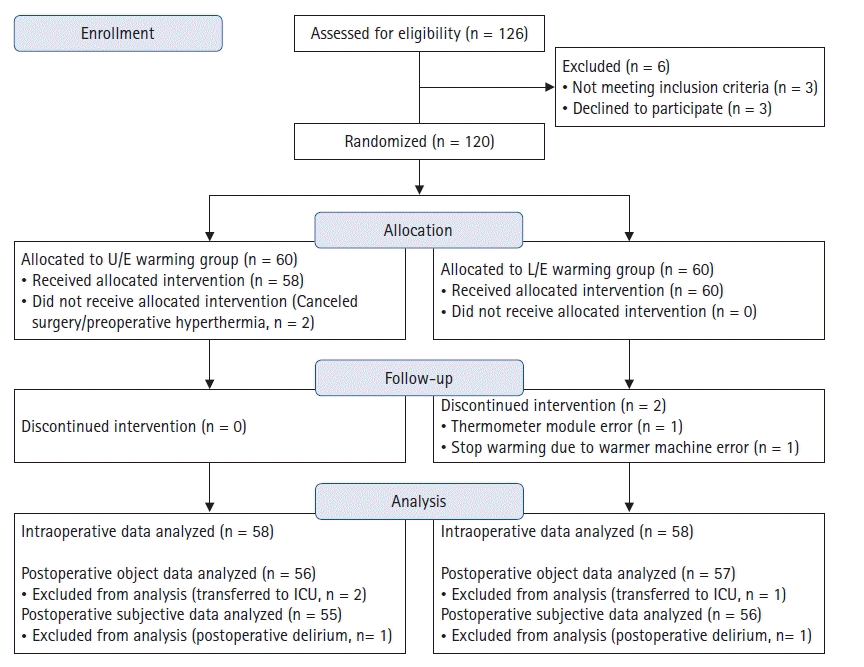 | Fig. 1.CONSORT diagram. ICU: intensive care unit. 
|
The baseline characteristics of the patients, and the level of spine surgery, duration of anesthesia, duration of the unwarmed period (from arrival in the operating room to the start of intraoperative warming), body temperature upon arrival in the pre-anesthetic holding area, ambient temperature of the operating room and PACU, and fluid volume are shown in
Table 1. No clinically significant differences between the two groups in these characteristics were observed (
Table 1).
Table 1.
Baseline Patient Characteristics
|
Variable |
Upper-warming group (n = 58) |
Lower-warming group (n = 58) |
P value |
|
Age (yr) |
69 (59.8, 77.0) |
69 (59.8, 76.3) |
0.840 |
|
Sex (M/F) |
24/34 |
26/32 |
0.708 |
|
Weight (kg) |
62.6 ± 11.17 |
65.1 ± 12.99 |
0.266 |
|
Height (cm) |
158.3 ± 8.0 |
160.1 ± 9.3 |
0.276 |
|
BMI (kg/m2) |
24.9 ± 3.49 |
25.2 ± 3.12 |
0.592 |
|
BSA (m2) |
1.631 ± 0.171 |
1.681 ± 0.203 |
0.152 |
|
ASA-PS classification (Ⅰ/Ⅱ/Ⅲ) |
11/33/14 |
16/33/9 |
0.366 |
|
Level of spine surgery |
|
|
0.787 |
|
Discectomy |
7 (12.1) |
7 (12.1) |
|
|
PD only |
19 (32.8) |
19 (32.8) |
|
|
Single-level PLIF and PD |
22 (37.9) |
20 (34.5) |
|
|
Two-level PLIF and PD |
4 (6.9) |
8 (13.8) |
|
|
Multilevel lumbar surgery (> 2 levels)*
|
3 (5.2) |
1 (1.7) |
|
|
Expanded to thoracic level (> 2 levels)*,†
|
3 (5.2) |
3 (5.2) |
|
|
Duration of anesthesia (min) |
183 (130.0, 250.0) |
200 (123.8, 289.8) |
0.469 |
|
Duration of unwarmed period (min)‡
|
38 ± 10.5 |
38 ± 10.6 |
0.951 |
|
Initial body temperature (°C) |
37.0 ± 0.37 |
36.9 ± 0.34 |
0.570 |
|
OR temperature (°C) |
21.3 ± 0.94 |
21.3 ± 0.95 |
0.713 |
|
PACU temperature (°C)§
|
26.0 (24.85, 26.48) |
25.8 (24.75, 26.50) |
0.598 |
|
Fluid volume (ml) |
600 (300, 1225) |
750 (300, 1163) |
0.474 |

The incidence of intraoperative hypothermia, was significantly lower in the upper-warming group than in the lower-warming group (55.2% vs. 75.9%, OR 0.392 [0.177, 0.866], P = 0.019). The severity of intraoperative hypothermia differed significantly between the two groups (P = 0.018) (
Table 2). The incidence of immediate postoperative hypothermia in the PACU was lower in the upper-warming group than in the lower-warming group (21.4% vs. 49.1%, OR 0.282 [0.124, 0.643], P = 0.002) (
Table 2).
Table 2.
Intraoperative and Postoperative Outcomes
|
Variable |
Upper-warming group |
Lower-warming group |
Odds ratio (95% CI) |
P value |
|
Intraoperative variables |
(n = 58) |
(n = 58) |
|
|
|
Intraoperative hypothermia |
32 (55.2) |
44 (75.9) |
0.392 (0.177, 0.866) |
0.019 |
|
Severity of intraoperative hypothermia |
|
|
|
0.018 |
|
Mild (35.5–36.0°C) |
20 (34.5) |
21 (36.2) |
|
|
|
Moderate (35.0–35.4°C) |
8 (13.8) |
9 (15.5) |
|
|
|
Severe (34.5–34.9°C) |
3 (5.2) |
10 (17.2) |
|
|
|
Very severe (< 34.5°C) |
0 (0.0) |
4 (6.9) |
|
|
|
Duration of intraoperative hypothermia (min) |
20.0 (0, 112.5) |
102.5 (11.3, 186.3) |
|
0.005 |
|
Blood loss (ml) |
150 (93, 320) |
200 (100, 400) |
|
0.241 |
|
Transfusion (n) |
0 |
2 (3.4) |
|
0.496 |
|
Objective PACU variables |
(n = 56) |
(n = 57) |
|
|
|
PACU hypothermia |
12 (21.4) |
28 (49.1) |
0.282 (0.124, 0.643) |
0.002 |
|
Shivering score (0/1/2) |
53/3/0 |
53/4/0 |
|
1.000 |
|
LOS at PACU (min) |
37 (34.3, 45.0) |
40 (34.0, 47.5) |
|
0.296 |
|
Subjective PACU variables |
(n = 55) |
(n = 56) |
|
|
|
Highest TCS |
50 (50, 50) |
50 (50, 50) |
|
0.808 |
|
Lowest TCS |
50 (50, 50) |
50 (50, 50) |
|
0.073 |
|
Patient satisfaction (4/3/2) |
32/21/2 |
29/22/5 |
|
0.485 |

Intraoperative blood loss and the transfusion requirement did not differ between the two groups (P = 0.241 and P = 0.496, respectively). Postoperative shivering score (P = 1.000) and the highest and lowest thermal comfort scale scores (P = 0.808 and P = 0.073, respectively) in the PACU also did not differ significantly between the groups. Satisfaction with the warming protocol did not differ between the groups (P = 0.485), nor did the length of stay in the PACU (P = 0.296) (
Table 2). No adverse effects from forced-air warming, such as skin irritation or burns, were observed in any patient.
The change in body temperature over time differed significantly between the two groups (P < 0.001). The group difference from 75 min after induction of anesthesia to the end of recovery was significant according to the Bonferroni
post-hoc test at > 0.2°C (which has been defined as a significant clinical difference in hypothermic patients) [
15]. The significant decrease in body temperature compared with the preoperative temperature persisted throughout the recovery period. The greatest decrease in body temperature in the upper-warming group occurred 60 min after inducing anesthesia (0.98°C); it occurred after 135 min (1.23°C) in the lower-warming group (
Fig. 2).
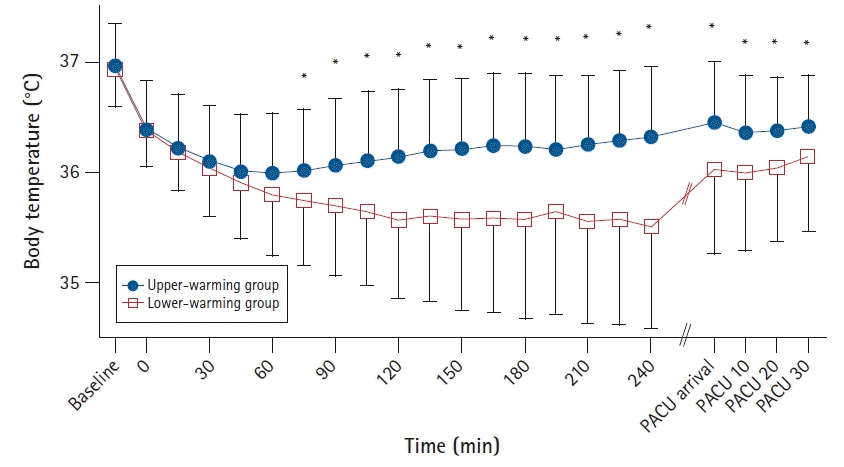 | Fig. 2.Perioperative body temperature. Error bars indicate ± 1 SD of temperature. Preoperative and postoperative core temperatures of the patients were measured using a tympanic membrane thermometer. The intraoperative core temperature measured using a nasopharyngeal probe was recorded every 15 min after anesthesia was induced. Significant drops in body temperature from baseline values were detected in both groups (P < 0.001). The temperature was higher in the upper-warming group beginning from 75 min after induction of anesthesia to the end of the PACU stay compared with the lower-warming group. *P < 0.05 for the group difference. Baseline: immediately after arrival in the pre-anesthetic holding area; intraoperative 0 min: immediately after inserting the nasopharyngeal probe; PACU arrival: immediately after arrival in the PACU; PACU 10, 20, and 30 min: 10, 20, and 30 min after arrival in the PACU. SD: standard deviation, PACU: post-anesthesia care unit. 
|
Hosmer and Lemeshow’s goodness-of-fit test showed that the regression models for intraoperative hypothermia (< 36.0°C) and severe intraoperative hypothermia (< 35.0°C) were suitable (P = 0.704, P = 0.956, respectively), and the models were statistically significant (both P < 0.001). Six variables were independently related to intraoperative hypothermia. Upper-warming (OR 0.221 [0.072, 0.683], P = 0.009), high ambient temperature (OR 0.280 [0.143, 0.548], P < 0.001), and high preoperative body temperature (OR 0.027 [0.004, 0.164], P < 0.001) were protective against hypothermia; and ASA-PS 2–3 (compared with ASA-PS 1; OR 6.608 [1.138, 38.366], P = 0.035 and OR 6.118 [1.509, 24.806], P = 0.011, respectively), long anesthetic duration (OR 1.009 [1.003, 1.016], P = 0.005), and high BSA/weight ratio (OR 1.659 [1.225, 2.247], P = 0.001) were independent risk factors for intraoperative hypothermia (
Fig. 3).
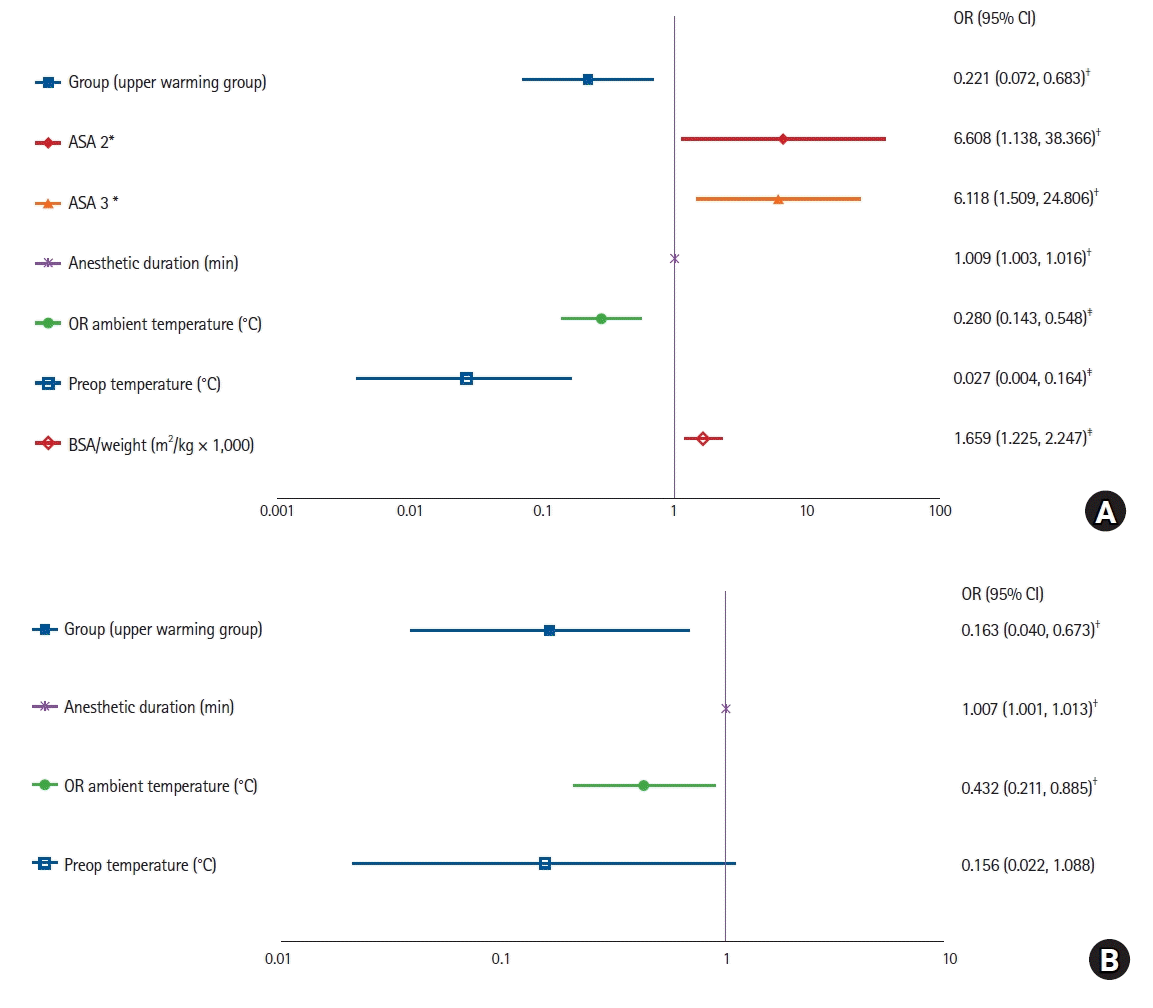 | Fig. 3.Risk factors for intraoperative hypothermia (< 36.0°C) and severe intraoperative hypothermia (< 35.0°C). (A) The risk factors for intraoperative hypothermia (< 36.0°C) and (B) severe intraoperative hypothermia (< 35.0°C). Multivariable logistic regression analysis was performed post-hoc to identify variables affecting intraoperative hypothermia and severe intraoperative hypothermia. Eleven variables (BSA/weight × 1,000, group, sex, age, ASA-PS classification, anesthetic duration, surgery type [> 2 levels], interval duration, OR ambient temperature, preoperative temperature, fluid administration [> 1,000 ml]) were included as independent variables in the multivariable logistic regression. The backward stepwise elimination method was applied to select variables based on the log-likelihood ratio. *ASA-PS risk classification compared with ASA-PS 1. †P < 0.05. ‡P < 0.001. ASA-PS: American Society of Anesthesiologists physical status, BSA: body surface area, OR: odds ratio, OR ambient temperature: operating room ambient temperature. 
|
Four variables were included in the severe intraoperative hypothermia model, three of which were independently related to severe intraoperative hypothermia. Upper-extremity warming group (OR 0.163 [0.040, 0.673], P = 0.012) and high ambient temperature (OR 0.432 [0.211, 0.885], P = 0.022) were protective against severe hypothermia, and long anesthetic duration (OR 1.007 [1.001, 1.013], P = 0.028) was an independent risk factor for severe intraoperative hypothermia (
Fig. 3).
Go to :

Discussion
This study demonstrated that forced-air warming using an upper-body blanket prevented perioperative hypothermia more effectively than did warming of the lower body with a blanket during spine surgery in the prone position.
Previous studies have reported that warming the lower body is more effective than warming the upper body for patients in the supine position [
9,
10]. Motamed et al. [
9] reported that lower-body warming resulted in a greater initial redistribution of the core temperature but normothermia was regained more rapidly during major abdominal surgery (120 vs. 180 min). Yamakage et al. [
10] reported that lower-body warming below the T10 dermatome was more effective than upper-body warming above the T7 dermatome in patients receiving spinal anesthesia.
Brauer et al. [
4,
5] used a Cooper manikin model, and reported that the maximum heat transfer values were 18.3 and 26.6 W with lower- and upper-body blankets, respectively. However, the lower-body blanket covered a larger area (49 W) than the upper-body blanket (37.8 W). The total heat balance was approximately 10 W higher using the lower-body blanket [
4,
5].
Min et al. [
11] reported that upper-body warming was more effective than lower-body warming when the patient was in the lateral decubitus position during thoracoscopic surgery. They attributed this to padding between the legs, which reduced the surface area covered by the lower-body blanket. They also suggested that heat distribution inside the blanket may vary more with a larger lower-body blanket.
In this study, forced-air warming of the upper-body blanket prevented perioperative hypothermia more effectively than did warming of the lower-body blanket, with the patient in the prone position during spine surgery. This result may be explained as follows.
First, the BSA covered by the upper-body blanket was larger, (covering both arms and hands, and the entire trunk above the T4 spinous process level) than that covered by the lower-body blanket. The area covered by the upper-body blanket was similar to that reported by Brauer et al. (~0.35 m
2). The lower-body blanket covered both legs and the lower buttocks below the coccyx, but the lower abdomen was not covered (~0.24 m
2 in the study of Brauer et al.) [
4,
5]. The major reason for the difference in results between a previous study conducted in the supine position and our study may be that lower-body warming reached up to the T10 dermatome in the previous investigation [
10].
Second, heat transfer may be higher with the upper- versus lower-body blanket, as also reported by Brauer et al. [
4,
5]. They reported that the maximum heat transfer values were 18.3 and 26.6 W using lower- and upper-body blankets, respectively. Min et al. [
11] suggested that the larger size of the lower-body blanket might explain the variability in heat distribution and lower efficacy. However, we suggest that the blanket design (upper-body blanket: narrow and long, lower-body blanket: wide and short) and the location of the nozzle access could have been more important in our study because the upper- and lower-body blankets were the same size (208 × 71 cm [14,768 cm
2] and 104 × 142 cm [14,768 cm
2], respectively).
Third, in the upper-warming group, continuous monitoring and management of blanket inflation was better performed, because the forced-air warmer was located close to the anesthesiologist. However, in the lower-warming group, the warming blanket was remote from the anesthesiologist and the entire blanket was covered by a surgical drape, making monitoring and management of blanket inflation relatively difficult. In addition, although the surgeon was aware of the lower-warming, surgical instruments were often placed on the lower-body blanket during the spine surgery; this could explain the variable heat distribution. This may have affected the results of this study.
Fourth, the difference in distance between the warming and measurement sites may have affected the results. We took the nasopharyngeal temperature as the intraoperative core temperature; the nasopharyngeal temperature is more reliable than the bladder temperature because the latter is strongly influenced by urine flow [
16,
17]. An esophageal probe may be misplaced due to the relatively long distance from the incisor (approximately 40 cm) compared to a nasopharyngeal probe [
14,
18–
20]. However, the upper-body blanket and nasopharynx are closer together compared with the nasopharynx and lower-body blanket, which could raise the nasopharyngeal temperature more quickly, even though the head was not covered with the upper-body blanket in our study. Further studies evaluating the effect on the temperature measurement site of the warming site are needed.
One study reported on the differences between upper- and lower-body blanket warming during spine surgery in the prone position. Buraimoh et al. [
12] reported no difference in warming efficacy between upper- and lower-body blankets in patients undergoing spine surgery. In their study, the incidence rates of severe hypothermia (< 35°C) and mild to moderate hypothermia (35–36°C) in the upper-warming group were similar to those in our study (18.4% and 34.2%, respectively), but the rates of severe and mild to moderate hypothermia in the lower-warming group were lower than our rates (11.1% and 30.6%, respectively). This was probably because the BSA covered by the lower-body warming blanket used in the previous study (an underbody warming blanket that covered the torso and legs) was larger than in our study. In addition, the use of bladder temperature might have affected the results. Forced-air warming underbody blankets have been shown to be effective, but are more expensive than conventional warming devices and so are not particularly popular [
8]. Our findings may help with blanket selection.
Reduced perioperative complications and enhanced patient satisfaction due to the lower incidence of hypothermia were not confirmed in our upper-warming group because of the low power of the study. The lack of any differences in intraoperative blood loss and the transfusion requirement may have been partially influenced by the minimally invasive surgery that was performed. The lack of any differences in postoperative shivering, thermal discomfort, and patient satisfaction may have been due to the continuous full-body warming in the PACU in both groups.
In our study, heat redistribution within the first hour was greater than that in a previous study of spine surgery patients [
21]. Differences in participant characteristics and warming blankets may explain this result. We included only patients undergoing thoracolumbar spine surgery, in whom the center of the body is more widely exposed, while the previous study included cervical spine surgical patients who were warmed using a full-body blanket or spine-specific blanket [
21]. The long unwarmed period (38 min) in our study could explain the greater decrease in core temperature.
Our results show that a higher ASA-PS, lower preoperative body temperature, and large surface area to body mass ratio were independent risk factors for intraoperative hypothermia. The effect of a low preoperative body temperature has been reported in previous studies [
22,
23]. The effect of a large surface area to body mass ratio on thermoregulation and the relationship between the surface area to body mass and ambient temperature have been documented [
24]. Therefore, it is intuitive that a large surface area to body mass ratio was an independent risk factor for intraoperative hypothermia in this study. A long anesthetic duration and low ambient temperature were independent risk factors for intraoperative hypothermia and severe intraoperative hypothermia, consistent with previous studies [
22,
23]. Therefore, we suggest that a high ambient temperature can prevent severe hypothermia, and additional warming effort is required if anesthesia of long duration is anticipated.
This study had some limitations. First, the tympanic temperature was measured to help reduce discomfort in conscious patients, but the pre- and post-operative tympanic temperatures can differ from the intraoperative nasopharyngeal temperature. Nevertheless, tympanic temperature is the most accurate and precise peripheral temperature measurement [
13]. Second, for the reasons mentioned above, the nasopharyngeal temperature was taken as the core temperature in our study [
16]. We placed the nasopharyngeal temperature probe at a depth of 9–10 cm in the nasopharynx based on a previous imaging study, and the probe was fixed with tape so that its position did not change [
14]. However, the prone position could have resulted in changes in the probe position and nasal secretions. Third, the results may not be informative regarding the effect of the level, location, or type of spine surgery, because the patients showed no differences in surgical characteristics. Also, the results may not generalize to patients undergoing major thoracolumbar spine surgery, as most of our patients underwent spine surgery below level 2. Further studies examining additional surgical factors are required. Fourth, no follow-up was performed, so long-term complications are unknown. Randomized controlled trials including larger samples and evaluating long-term complications of mild perioperative hypothermia are required.
In conclusion, upper-body blanket warming was more effective than lower-body blanket warming to prevent perioperative hypothermia during thoracolumbar spine surgery in the prone position.
Go to :







 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download