Abstract
As coronavirus disease 2019 (COVID-19) has spread worldwide, the rate of COVID-19 vaccination uptake is encouraging. Neurological complications associated with COVID-19 vaccines such as stroke, Guillain-Barré syndrome, and Bell’s palsy have been reported. Recently, late-onset myasthenia gravis (MG) following COVID-19 vaccination has been reported. To date, however, there has been no evidence of increased risk of early-onset MG following COVID-19. Here, we report a case of a patient with new-onset MG that arose after receiving a COVID-19 vaccine. A 33-year-old woman suddenly experienced generalized weakness and diplopia on the evening she had received the second dose of the Pfizer-BioNTech COVID-19 vaccine. The temporal relationship suggests that this new-onset MG is related to the vaccination. It also implies that COVID-19 vaccination could trigger early-onset MG symptoms in patients at risk of MG.
Go to : 
Graphical Abstract
Go to : 
Myasthenia gravis (MG) is an autoimmune neuromuscular junction disorder characterized by production of antibodies against the acetylcholine receptor (AchR).1 Since these antibodies impair the function of the AchR, the efforts to contract muscles exacerbate muscle weakness, which improves with rest.2 It is known that infection, medication or vaccination can exacerbate the symptoms of MG. A few studies have reported a relationship between MG and active coronavirus disease 2019 (COVID-19) infection, or medications used to treat COVID-19 infection.345 However, there has been no evidence of increased risk of early-onset MG following COVID-19 vaccination. We report a case of a patient with early-onset MG induced by COVID-19 vaccination.
Go to : 
A 33-year-old woman without any significant previous medical history presented to our emergency department due to bilateral ptosis and binocular diplopia following the second dose of the Pfizer-BioNTech COVID-19 vaccine. On the evening of her second vaccination, she suddenly felt generalized weakness, myalgia, and diplopia. Three days after receiving this vaccine, she developed bilateral ptosis. Four days after symptom onset, she experienced difficulty in raising her arms and moving her neck. Those symptoms showed a diurnal fluctuation. On neurologic examination, decreased muscle strength was noted as medical research council (MRC) grade 4/5 in, both upper and lower extremities. Bilateral ptosis, binocular diplopia, dysarthria, and dysphagia were also identified. There was no sensory involvement, and the patient’s deep tendon reflexes were reduced.
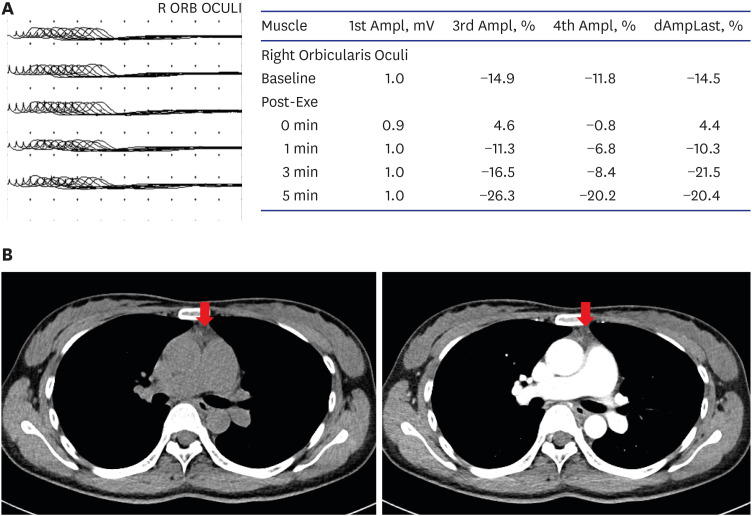
MG was suspected and was confirmed using a neostigmine test and typical electromyographic findings. Her symptoms temporarily improved after the neostigmine test. The initial quantitative MG score was 14 points for dysphagia, dysarthria, and weakness of both the upper and lower extremities. Forty-five minutes after administration of intramuscular neostigmine, the quantitative MG score had improved to 5 points. Her diplopia, dysphagia, and bilateral limb weakness recovered fully. A repetitive nerve stimulation test (RNST) revealed a significant decrement response in the right orbicularis oculi (Fig. 1A). The patient’s serum AchR antibody titer was < 0.02 nmol/L, and her muscle-specific kinase antibody titer was < 0.01 nmol/L; both within the normal ranges. Motor and sensory nerve conduction studies were normal. Mild thymic hyperplasia was observed on chest computed tomography (Fig. 1B).
We finally diagnosed the patient with double-seronegative MG due to the combination of variability in symptoms, response to neostigmine, and typical electromyographic findings. Based on her clinical features and normal brain computed tomography and nerve conduction studies, we ruled out other diagnoses, such as other neuromuscular junction diseases, acute inflammatory demyelinating polyneuropathy, myopathy, brain stem ischemia, and motor neuron disease. She received oral pyridostigmine 360 mg/day from the day of admission. Her ptosis, dysarthria, and muscle strength were partially improved. On the fourth day of hospitalization, we were planning to initiate immunotherapy such as a corticosteroid or intravenous immunoglobulin. However, the patient chose to be discharged.
Go to : 
The present patient experienced ocular symptoms, a few hours after receiving the second dose of a COVID-19 vaccine; the symptoms temporarily improved with neostigmine administration. The temporal relationship between COVID-19 vaccination and the development of symptoms suggests that this new-onset MG is related to the vaccination. Although the underlying pathogenesis is unclear, it is thought that changes that take place in the immune response after vaccination could elicit the production of antibodies against AchR. To date, neurological side effects reported following COVID-19 vaccination have included hemorrhagic stroke, cerebral venous thrombosis, Guillain-Barré syndrome, and Bell’s palsy. Chavez et al.6 recently described one case of MG associated with the first dose of a COVID-19 vaccine. The patient was diagnosed with late-onset MG and he showed an unusually rapid progression despite appropriate management. In contrast, our patient was a 33-year-old female with thymic hyperplasia, which is characteristic of early-onset MG. This is the first case report of early-onset MG following a Pfizer-BioNTech COVID-19 vaccine and implies that COVID-19 vaccination can trigger early-onset MG in at-risk patients. In addition, in our case, AchR and MuSK antibodies were absent in the serum. The majority of patients with thymoma or thymic hyperplasia associated MG test positive for AchR antibodies. Only a few case reports of seronegative MG associated with thymoma have been reported.7 We suspect that our patient has novel antibodies or antibody levels that are undetectable using the currently available assay methods. Our case broadens the clinical presentations related to double seronegative MG.
A few studies have reported new-onset MG after administration of vaccines against influenza, hepatitis B, and human papillomavirus.891011 These patients have exhibited ocular and/or bulbar symptoms. The time interval between vaccination and symptom onset has ranged from three days to six weeks. These patients received treatment with oral pyridostigmine and prednisolone and showed a good prognosis. Even though the incidence remains very low, clinicians should be aware of development or exacerbation of MG following vaccination.
COVID-19 can cause respiratory disease leading to high mortality and morbidity. Because of their underlying immunocompromised state along with superimposed respiratory or bulbar weakness, MG patients are at risk for developing severe acute respiratory distress syndrome and could have a higher mortality rate when infected with COVID-19.3 In the “CARE-MG” study, which included 91 MG patients infected with COVID-19, 36 (40%) patients reported exacerbation of their MG symptoms that required rescue therapy, and 22 (24%) died due to COVID-19.4 Therefore, it is important to monitor the safety of currently approved vaccines and to determine whether vaccination is superior to active COVID-19 infection in MG patients. Furthermore, as COVID-19 has spread worldwide and the rate of COVID-19 vaccination is encouraging, the importance of further studies in MG patients is increasing.
Go to : 
References
1. Lindstrom JM, Seybold ME, Lennon VA, Whittingham S, Duane DD. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology. 1976; 26(11):1054–1059. PMID: 988512.

2. Hehir MK, Silvestri NJ. Generalized myasthenia gravis: classification, clinical presentation, natural history, and epidemiology. Neurol Clin. 2018; 36(2):253–260. PMID: 29655448.
3. Camelo-Filho AE, Silva AM, Estephan EP, Zambon AA, Mendonça RH, Souza PV, et al. Myasthenia gravis and COVID-19: clinical characteristics and outcomes. Front Neurol. 2020; 11:1053. PMID: 33013676.

4. Muppidi S, Guptill JT, Jacob S, Li Y, Farrugia ME, Guidon AC, et al. COVID-19-associated risks and effects in myasthenia gravis (CARE-MG). Lancet Neurol. 2020; 19(12):970–971. PMID: 33212055.

5. Anand P, Slama MC, Kaku M, Ong C, Cervantes-Arslanian AM, Zhou L, et al. COVID-19 in patients with myasthenia gravis. Muscle Nerve. 2020; 62(2):254–258. PMID: 32392389.
6. Chavez A, Pougnier C. A case of COVID-19 vaccine associated new diagnosis myasthenia gravis. J Prim Care Community Health. 2021; 12:21501327211051933. PMID: 34709075.

7. Maggi L, Andreetta F, Antozzi C, Confalonieri P, Cornelio F, Scaioli V, et al. Two cases of thymoma-associated myasthenia gravis without antibodies to the acetylcholine receptor. Neuromuscul Disord. 2008; 18(8):678–680. PMID: 18657424.

8. Takizawa T, Kojima M, Suzuki S, Osada T, Kitagawa S, Nakahara J, et al. New onset of myasthenia gravis after intravesical Bacillus Calmette-Guerin: a case report and literature review. Medicine (Baltimore). 2017; 96(46):e8757. PMID: 29145329.
9. Bahri ME, Louzir B, Othmani S, Battikh R, Bahri MO. FRI0248 Myasthenia Gravis after Hepatitis B Vaccine. Report of One Case. London, UK: BMJ Publishing Group Ltd;2001.
10. Stübgen JP. Neuromuscular disorders associated with hepatitis B vaccination. J Neurol Sci. 2010; 292(1-2):1–4. PMID: 20207367.

11. Chung JY, Lee SJ, Shin BS, Kang HG. Myasthenia gravis following human papillomavirus vaccination: a case report. BMC Neurol. 2018; 18(1):222. PMID: 30593270.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download