A 9-year-old boy was admitted to our hospital on July 26, 2021, and diagnosed with asymptomatic COVID-19. He was under self-quarantine for 6 days due to close contact with a confirmed case. A reverse-transcriptase quantitative real-time polymerase chain reaction (PCR) assay using a PowerChek™ SARS-CoV-2 S-gene mutation detection kit (version 1, P681; Kogene Biotech, Seoul, Republic of Korea) was used to diagnose the SARS-CoV-2 delta variant. Relevant medication history included anti-epileptic medication for Lennox-Gastaut syndrome, including levetiracetam, lamotrigine, zonisamide, sodium valproate, clobazam, and cannabidiol, beginning at 8 months of age. Even though the patient had partial seizure events about twice a month and a developmental delay, he was able to move on his own and take food orally. No respiratory support was needed before having COVID-19. On admission, there were no COVID-19-associated symptoms, such as fever, cough, sputum, or dyspnea. His body temperature was 36.7°C, the heart rate was 109 bpm, the respiratory rate was 21 breaths per minute, and the saturation of percutaneous oxygen (SpO
2) was sustained at ≥ 95% with room air. His height was 130 cm (5th percentile), weight was 35 kg (50th percentile), and the body mass index was 20.7 kg/m
2 (75th percentile). The initial laboratory findings were as follows: white blood cell count 12,590/μL, hemoglobin 11.5 g/dL, platelet count 473,000/μL, C-reactive protein (CRP) 2.74 mg/L (range, 0–5), and D-dimer 0.48 mg/L (range, 0–0.5). The results of initial venous blood gas analysis were within the normal ranges (pH 7.40, pCO
2 = 40 mmHg, pO
2 = 44 mmHg, and HCO
3 = 24.8 mmHg). The initial chest X-ray was clear, without any COVID-19 lesions in either lung (
Fig. 1A). A rhinovirus was detected on the PCR performed to determine co-infection. Since there was no negative pressure room in our hospital, the sputum culture test could not be performed. No bacteria were detected on the blood culture.
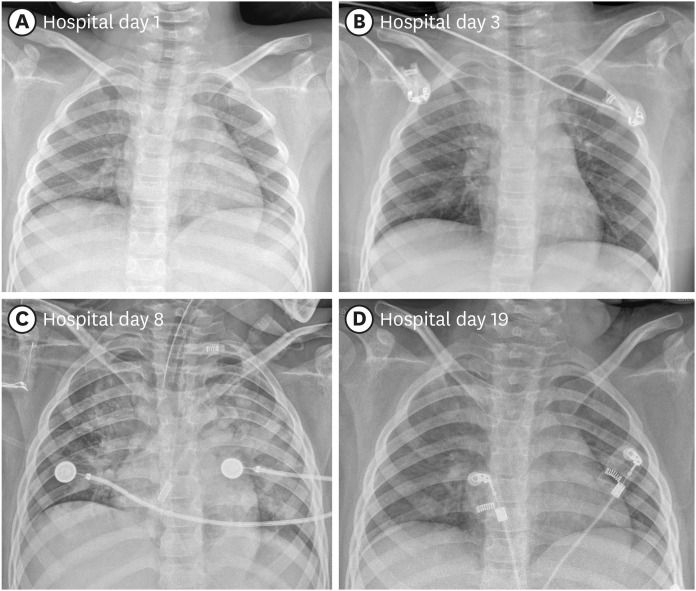 | Fig. 1 Chest X-ray images of the patient. (A) Upon admission. (B) Right upper lobe haziness following intractable seizures on post-admission day 3. (C) Increased opacity in both lungs on post-admission day 8 when PaO2/FiO2 decreased to 84.3. (D) Chest X-ray at the time of discharge on post-admission day 19.
|
On post-admission day 3, a high fever of 39.8°C developed with intractable tonic-clonic seizures that lasted for about two hours and persisted despite repeated administration of lorazepam. The SpO
2 decreased to 76% despite O
2 supplementation of 10 L/min through an O
2 reserve mask. The patient was transferred to the COVID intensive care unit following administration of a loading dose of phenytoin and midazolam, along with intubation. Upon intubation, saturation was maintained at ≥ 95% with ventilator care at an FiO
2 of 21%; at this time, a newly developed area of increased density was observed on the right upper lobe (
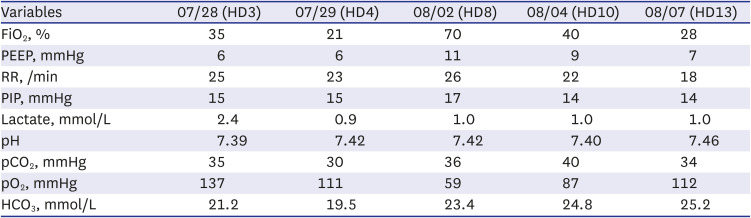
Fig. 1B). The ventilator settings on the day of intubation and on the next day, as well as the results of atrial blood gas analysis, are displayed on
Table 1. Empirical antibiotics were initiated using piperacillin-tazobactam due to the potential for aspiration pneumonia. The following day, the oxygen requirement increased, and increased haziness was observed on both lung fields, particularly on the right upper lobe. Laboratory findings indicated elevated CRP (120.4 mg/L), and corticosteroids were administered twice (methylprednisolone, 1 mg/kg/dose). Empirical antibiotics were changed to vancomycin and meropenem. Despite the conservative management of COVID-19, oxygenation continued to deteriorate. On the eighth day of admission, PaO
2/FiO
2 decreased to 84.3 (
Table 1), and both lungs exhibited increased consolidation on chest X-ray (
Fig. 1C). The laboratory findings at that time were as follows: white blood cell count 7,710/μL, lymphocytes 1,150/μL, hemoglobin 9.1 g/dL, platelet count 209,000/μL, CRP 63.98 mg/L, aspartate aminotransferase 59 IU/L, and alanine aminotransferase 48 IU/L. The patient had persistent, recurrent fever. To improve oxygenation, a high positive end-expiratory pressure (11 mmHg) and low tidal volume (4–6 mL/kg) were set. A short course of methylprednisolone pulse therapy (10 mg/kg/day divided into two doses) was administered for two days and tapered over 11 days, which improved oxygenation with a PaO
2/FiO
2 of 245 (
Table 1). Furthermore, the pneumonic consolidation improved slightly in both lungs. Corticosteroids were tapered, and extubation was successful after 10 days of tracheal intubation. No side effects were reported, including hyperglycemia and hypertension. There was also no other major organ dysfunction. On post-admission day 27, the patient was discharged without O
2 requirement, and no remnant lesions were observed in either lung (
Fig. 1D).
Table 1
The ventilator setting and results of arterial blood gas analysis of the patient throughout the treatment by date
|
Variables |
07/28 (HD3) |
07/29 (HD4) |
08/02 (HD8) |
08/04 (HD10) |
08/07 (HD13) |
|
FiO2, % |
35 |
21 |
70 |
40 |
28 |
|
PEEP, mmHg |
6 |
6 |
11 |
9 |
7 |
|
RR, /min |
25 |
23 |
26 |
22 |
18 |
|
PIP, mmHg |
15 |
15 |
17 |
14 |
14 |
|
Lactate, mmol/L |
2.4 |
0.9 |
1.0 |
1.0 |
1.0 |
|
pH |
7.39 |
7.42 |
7.42 |
7.40 |
7.46 |
|
pCO2, mmHg |
35 |
30 |
36 |
40 |
34 |
|
pO2, mmHg |
137 |
111 |
59 |
87 |
112 |
|
HCO3, mmol/L |
21.2 |
19.5 |
23.4 |
24.8 |
25.2 |







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download