1. Carson JL, Kelley MA, Duff A, Weg JG, Fulkerson WJ, Palevsky HI, et al. The clinical course of pulmonary embolism. N Engl J Med. 1992; 326(19):1240–1245. PMID:
1560799.

2. Mayo J, Thakur Y. Pulmonary CT angiography as first-line imaging for PE: image quality and radiation dose considerations. AJR Am J Roentgenol. 2013; 200(3):522–528. PMID:
23436840.

3. Mayo J, Thakur Y. Acute pulmonary embolism: from morphology to function. Semin Respir Crit Care Med. 2014; 35(1):41–49. PMID:
24481758.

4. Remy-Jardin M, Pistolesi M, Goodman LR, Gefter WB, Gottschalk A, Mayo JR, et al. Management of suspected acute pulmonary embolism in the era of CT angiography: a statement from the Fleischner Society. Radiology. 2007; 245(2):315–329. PMID:
17848685.

5. Halpern EJ. Triple-rule-out CT angiography for evaluation of acute chest pain and possible acute coronary syndrome. Radiology. 2009; 252(2):332–345. PMID:
19703877.

6. Weikert T, Winkel DJ, Bremerich J, Stieltjes B, Parmar V, Sauter AW, et al. Automated detection of pulmonary embolism in CT pulmonary angiograms using an AI-powered algorithm. Eur Radiol. 2020; 30(12):6545–6553. PMID:
32621243.

7. Soffer S, Klang E, Shimon O, Barash Y, Cahan N, Greenspana H, et al. Deep learning for pulmonary embolism detection on computed tomography pulmonary angiogram: a systematic review and meta-analysis. Sci Rep. 2021; 11(1):15814. PMID:
34349191.

8. Fedullo P, Kerr KM, Kim NH, Auger WR. Chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2011; 183(12):1605–1613. PMID:
21330453.

9. Tunariu N, Gibbs SJ, Win Z, Gin-Sing W, Graham A, Gishen P, et al. Ventilation-perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. J Nucl Med. 2007; 48(5):680–684. PMID:
17475953.

10. Wang M, Wu D, Ma R, Zhang Z, Zhang H, Han K, et al. Comparison of V/Q SPECT and CT angiography for the diagnosis of chronic thromboembolic pulmonary hypertension. Radiology. 2020; 296(2):420–429. PMID:
32427559.

11. Hong YJ, Shim J, Lee SM, Im DJ, Hur J. Dual-energy CT for pulmonary embolism: current and evolving clinical applications. Korean J Radiol. 2021; 22(9):1555–1568. PMID:
34448383.

12. Im DJ, Hur J, Han K, Suh YJ, Hong YJ, Lee HJ, et al. Prognostic value of dual-energy CT-based iodine quantification versus conventional CT in acute pulmonary embolism: a propensity-match analysis. Korean J Radiol. 2020; 21(9):1095–1103. PMID:
32691545.

13. Zhang LJ, Chai X, Wu SY, Zhao YE, Hu XB, Hu YX, et al. Detection of pulmonary embolism by dual energy CT: correlation with perfusion scintigraphy and histopathological findings in rabbits. Eur Radiol. 2009; 19(12):2844–2854. PMID:
19657658.

14. Boroto K, Remy-Jardin M, Flohr T, Faivre JB, Pansini V, Tacelli N, et al. Thoracic applications of dual-source CT technology. Eur J Radiol. 2008; 68(3):375–384. PMID:
18929452.

15. Fink C, Johnson TR, Michaely HJ, Morhard D, Becker C, Reiser M, et al. Dual-energy CT angiography of the lung in patients with suspected pulmonary embolism: initial results. RoFo Fortschr Geb Rontgenstr Nuklearmed. 2008; 180(10):879–883. PMID:
19238637.

16. Pontana F, Faivre JB, Remy-Jardin M, Flohr T, Schmidt B, Tacelli N, et al. Lung perfusion with dual-energy multidetector-row CT (MDCT): feasibility for the evaluation of acute pulmonary embolism in 117 consecutive patients. Acad Radiol. 2008; 15(12):1494–1504. PMID:
19000866.
17. Henzler T, Fink C, Schoenberg SO, Schoepf UJ. Dual-energy CT: radiation dose aspects. AJR Am J Roentgenol. 2012; 199(5):Suppl. S16–S25. PMID:
23097163.

18. Ciccotosto C, Goodman LR, Washington L, Quiroz FA, Indirect CT. Indirect CT venography following CT pulmonary angiography: spectrum of CT findings. J Thorac Imaging. 2002; 17(1):18–27. PMID:
11828208.
19. Yavas US, Calisir C, Ozkan IR. The interobserver agreement between residents and experienced radiologists for detecting pulmonary embolism and DVT with using CT pulmonary angiography and indirect CT venography. Korean J Radiol. 2008; 9(6):498–502. PMID:
19039265.

20. Kulkarni NM, Sahani DV, Desai GS, Kalva SP. Indirect computed tomography venography of the lower extremities using single-source dual-energy computed tomography: advantage of low-kiloelectron volt monochromatic images. J Vasc Interv Radiol. 2012; 23(7):879–886. PMID:
22633619.

21. Ma CL, Chen XX, Lei YX, Zhang XR, Jia YJ, Tian X, et al. Clinical value of dual-energy spectral imaging with adaptive statistical iterative reconstruction for reducing contrast medium dose in CT portal venography: in comparison with standard 120-kVp imaging protocol. Br J Radiol. 2016; 89(1062):20151022. PMID:
27031376.

22. Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011; 123(16):1788–1830. PMID:
21422387.

23. Sista AK, Kuo WT, Schiebler M, Madoff DC. Stratification, imaging, and management of acute massive and submassive pulmonary embolism. Radiology. 2017; 284(1):5–24. PMID:
28628412.

24. Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest. 2002; 121(3):877–905. PMID:
11888976.
25. Belenkie I, Dani R, Smith ER, Tyberg JV. The importance of pericardial constraint in experimental pulmonary embolism and volume loading. Am Heart J. 1992; 123(3):733–742. PMID:
1539525.

26. Ghaye B, Ghuysen A, Bruyere PJ, D'Orio V, Dondelinger RF. Can CT pulmonary angiography allow assessment of severity and prognosis in patients presenting with pulmonary embolism? What the radiologist needs to know. Radiographics. 2006; 26(1):23–39. PMID:
16418240.

27. Aujesky D, Obrosky DS, Stone RA, Auble TE, Perrier A, Cornuz J, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005; 172(8):1041–1046. PMID:
16020800.

28. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014; 35(43):3033–3069. PMID:
25173341.
29. Renard B, Remy-Jardin M, Santangelo T, Faivre JB, Tacelli N, Remy J, et al. Dual-energy CT angiography of chronic thromboembolic disease: can it help recognize links between the severity of pulmonary arterial obstruction and perfusion defects? Eur J Radiol. 2011; 79(3):467–472. PMID:
20488639.

30. Roberge G, Carrier M. How to manage patients with symptomatic subsegmental pulmonary embolism? Pol Arch Intern Med. 2020; 130(4):310–316. PMID:
32091505.

31. Yoo HH, Marin FL. Isolated subsegmental pulmonary embolism: current therapeutic challenges. Pol Arch Intern Med. 2020; 130(11):986–991. PMID:
32426959.
32. Ekici M, Ekici A, İleri Ş. Chronic CT features in PE patients with co-existing DVT. Am J Emerg Med. 2021; 46:126–131. PMID:
33744749.

33. Alias S, Redwan B, Panzenboeck A, Winter MP, Schubert U, Voswinckel R, et al. Defective angiogenesis delays thrombus resolution: a potential pathogenetic mechanism underlying chronic thromboembolic pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2014; 34(4):810–819. PMID:
24526692.
34. King MA, Ysrael M, Bergin CJ. Chronic thromboembolic pulmonary hypertension: CT findings. AJR Am J Roentgenol. 1998; 170(4):955–960. PMID:
9530043.

35. Chan AL, Juarez MM, Shelton DK, MacDonald T, Li CS, Lin TC, et al. Novel computed tomographic chest metrics to detect pulmonary hypertension. BMC Med Imaging. 2011; 11(1):7. PMID:
21447184.

36. Lin FY, Devereux RB, Roman MJ, Meng J, Jow VM, Jacobs A, et al. Cardiac chamber volumes, function, and mass as determined by 64-multidetector row computed tomography: mean values among healthy adults free of hypertension and obesity. JACC Cardiovasc Imaging. 2008; 1(6):782–786. PMID:
19356515.

37. Ascha M, Renapurkar RD, Tonelli AR. A review of imaging modalities in pulmonary hypertension. Ann Thorac Med. 2017; 12(2):61–73. PMID:
28469715.

38. Bankier AA, Janata K, Fleischmann D, Kreuzer S, Mallek R, Frossard M, et al. Severity assessment of acute pulmonary embolism with spiral CT: evaluation of two modified angiographic scores and comparison with clinical data. J Thorac Imaging. 1997; 12(2):150–158. PMID:
9179827.
39. Qanadli SD, El Hajjam M, Vieillard-Baron A, Joseph T, Mesurolle B, Oliva VL, et al. New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am J Roentgenol. 2001; 176(6):1415–1420. PMID:
11373204.
40. Mastora I, Remy-Jardin M, Masson P, Galland E, Delannoy V, Bauchart JJ, et al. Severity of acute pulmonary embolism: evaluation of a new spiral CT angiographic score in correlation with echocardiographic data. Eur Radiol. 2003; 13(1):29–35. PMID:
12541107.

41. van der Meer RW, Pattynama PM, van Strijen MJ, van den Berg-Huijsmans AA, Hartmann IJ, Putter H, et al. Right ventricular dysfunction and pulmonary obstruction index at helical CT: prediction of clinical outcome during 3-month follow-up in patients with acute pulmonary embolism. Radiology. 2005; 235(3):798–803. PMID:
15845793.

42. Wu AS, Pezzullo JA, Cronan JJ, Hou DD, Mayo-Smith WW. CT pulmonary angiography: quantification of pulmonary embolus as a predictor of patient outcome--initial experience. Radiology. 2004; 230(3):831–835. PMID:
14739314.

43. Collomb D, Paramelle PJ, Calaque O, Bosson JL, Vanzetto G, Barnoud D, et al. Severity assessment of acute pulmonary embolism: evaluation using helical CT. Eur Radiol. 2003; 13(7):1508–1514. PMID:
12835961.

44. Ghaye B, Ghuysen A, Willems V, Lambermont B, Gerard P, D’Orio V, et al. Severe pulmonary embolism:pulmonary artery clot load scores and cardiovascular parameters as predictors of mortality. Radiology. 2006; 239(3):884–891. PMID:
16603659.

45. Araoz PA, Gotway MB, Trowbridge RL, Bailey RA, Auerbach AD, Reddy GP, et al. Helical CT pulmonary angiography predictors of in-hospital morbidity and mortality in patients with acute pulmonary embolism. J Thorac Imaging. 2003; 18(4):207–216. PMID:
14561905.

46. Lualdi JC, Goldhaber SZ. Right ventricular dysfunction after acute pulmonary embolism: pathophysiologic factors, detection, and therapeutic implications. Am Heart J. 1995; 130(6):1276–1282. PMID:
7484782.

47. Smulders YM. Pathophysiology and treatment of haemodynamic instability in acute pulmonary embolism: the pivotal role of pulmonary vasoconstriction. Cardiovasc Res. 2000; 48(1):23–33. PMID:
11033105.

48. Pengo V, Lensing AW, Prins MH, Marchiori A, Davidson BL, Tiozzo F, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004; 350(22):2257–2264. PMID:
15163775.

49. Remy-Jardin M, Ryerson CJ, Schiebler ML, Leung AN, Wild JM, Hoeper MM, et al. Imaging of pulmonary hypertension in adults: a position paper from the Fleischner Society. Radiology. 2021; 298(3):531–549. PMID:
33399507.

50. Rashidi F, Parvizi R, Bilejani E, Mahmoodian B, Rahimi F, Koohi A. Evaluation of the incidence of chronic thromboembolic pulmonary hypertension 1 year after first episode of acute pulmonary embolism: a cohort study. Lung. 2020; 198(1):59–64. PMID:
31894412.

51. Asl Fallah S, Ghodsi S, Soleimani H, Mohebi M, Hossein Sabet A, Ariannejad H, et al. Incidence and predictors of chronic thromboembolic pulmonary hypertension following first episode of acute pulmonary embolism. Adv Respir Med. 2020; 88(6):539–547. PMID:
33393646.

52. Park JS, Ahn J, Choi JH, Lee HW, Oh JH, Lee HC, et al. The predictive value of echocardiography for chronic thromboembolic pulmonary hypertension after acute pulmonary embolism in Korea. Korean J Intern Med. 2017; 32(1):85–94. PMID:
27044855.

53. Lorenz G, Saeedan MB, Bullen J, Klok FA, Kroft LJ, Meijboom LJ, et al. CT-based biomarkers for prediction of chronic thromboembolic pulmonary hypertension after an acute pulmonary embolic event. AJR Am J Roentgenol. 2020; 215(4):800–806. PMID:
32809861.

54. Ende-Verhaar YM, Cannegieter SC, Vonk Noordegraaf A, Delcroix M, Pruszczyk P, Mairuhu AT, et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a contemporary view of the published literature. Eur Respir J. 2017; 49(2):1601792. PMID:
28232411.

55. Park SY, Lee SM, Shin JW, Choi BW, Kim H, Lee JS, et al. Epidemiology of chronic thromboembolic pulmonary hypertension in Korea: results from the Korean registry. Korean J Intern Med. 2016; 31(2):305–312. PMID:
26689916.

56. Brenot P, Jaïs X, Taniguchi Y, Garcia Alonso C, Gerardin B, Mussot S, et al. French experience of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Eur Respir J. 2019; 53(5):1802095. PMID:
31023842.

57. Ogawa A, Satoh T, Fukuda T, Sugimura K, Fukumoto Y, Emoto N, et al. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: results of a multicenter registry. Circ Cardiovasc Qual Outcomes. 2017; 10(11):e004029. PMID:
29101270.

58. Kawakami T, Ogawa A, Miyaji K, Mizoguchi H, Shimokawahara H, Naito T, et al. Novel angiographic classification of each vascular lesion in chronic thromboembolic pulmonary hypertension based on selective angiogram and results of balloon pulmonary angioplasty. Circ Cardiovasc Interv. 2016; 9(10):e003318. PMID:
27729418.

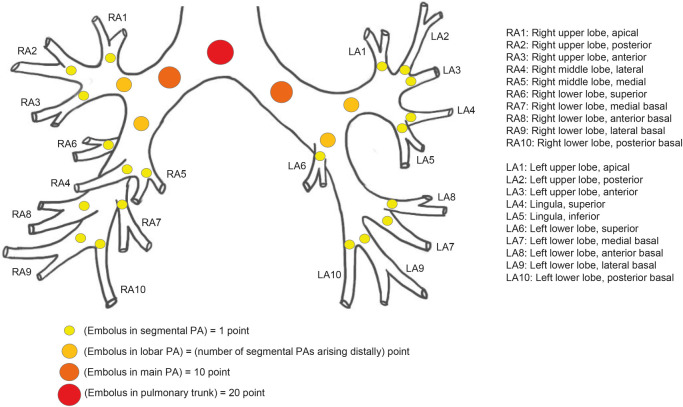

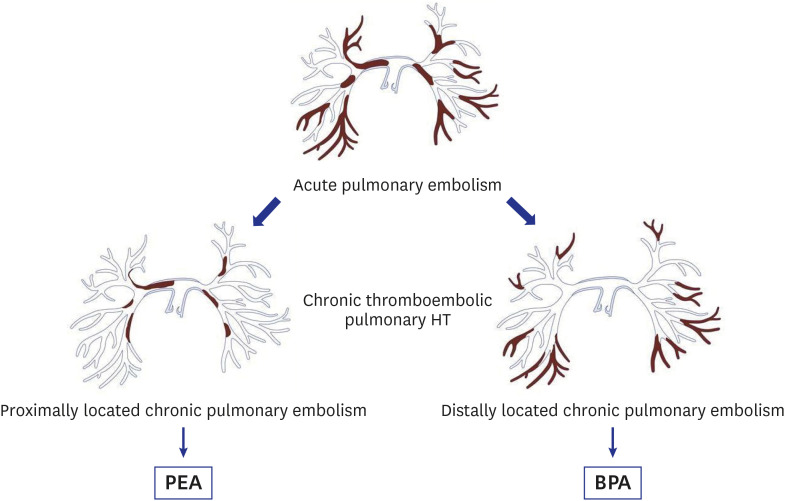
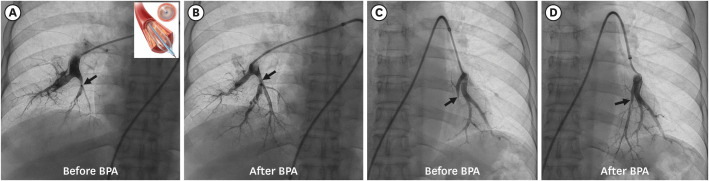
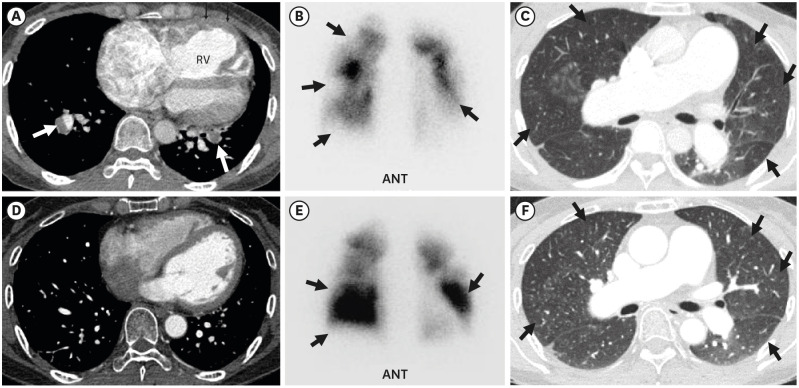




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download