A 70-year-old man presented at the emergency department with altered mental status. He was unable to perform the activities of daily living due to generalized weakness, diarrhea, and poor oral intake for seven days. He had hypertension, diabetes mellitus, diabetic retinopathy, chronic kidney disease (stage 3, baseline serum creatinine (Scr) was confirmed 1.68 mg/dL two years ago) and bipolar affective disorder treated with lithium. He was vaccinated with the ChAdOx1 nCoV-19 vaccine 10 days previously. His blood pressure was 158/114 mmHg and his body temperature was 37.0°C. Physical examination revealed drowsy mental status, dehydrated tongue, decreased skin turgor, and mild lower abdomen tenderness. Upon admission, his hemoglobin was 12.0 g/dL, the white blood cell count was 9,000/µL, platelet count was 204,000/µL, prothrombin time international normalized ratio (PT INR) was 1.08 (reference range, < 1.2), and d-dimer was 0.54 µg/mL (reference range, < 0.50). Serum blood urea nitrogen (39.0 mg/dL), Scr (3.52 mg/dL), C-reactive protein (10.43 mg/L; reference rage, 0–5.0 mg/L), and lithium levels (2.81 mEq/L; reference range, 0.6–1.2 mEq/L) were analyzed. The twenty-four-hour urine test showed a protein of 14.1 g and albumin of 11.1 g. There was marked low-density wall thickening of the cecum and ascending colon on the abdominopelvic computed tomography (CT) scan, compatible with infectious colitis. Diffusion-weighted magnetic resonance imaging of the brain and electroencephalogram were unremarkable. Under a diagnosis of lithium intoxication, hemodialysis was initiated. After six hemodialysis sessions (hospital day 2–5, 7, and 8) using heparin from the 4–6th session (hospital day 5, 7, 8), the lithium level decreased to below the toxic level (0.25 mEq/L) and the patient’s mental status recovered. SCr was decreased to 1.37 mg/dL. Nine days after the first heparin use, the patient’s platelet count abruptly decreased to 15,000/µL and his right calf was swollen. Ultrasonography showed venous thrombi in the right common femoral vein, right superficial femoral vein, and right anterior tibial vein. The patient had evidence of disseminated intravascular coagulation (DIC), including elevated D-dimer level, low fibrinogen levels, a mildly increased PT INR, and an activated partial thromboplastin time (
Table 1). Haptoglobin and total bilirubin levels were within the normal ranges. The ADAMTS 13 activity was 81.5%. The fluorescent antinuclear antibody test was positive (homogeneous pattern, 1:320). The extractable nuclear antigen (ENA) antibody panel including the antiphospholipid antibody and antiplatelet antibody were also negative. Despite the transfusion of platelet concentrates, the platelet counts remained low. Considering the deep vein thrombosis in combination with thrombocytopenia after COVID vaccination and also the recent exposure to heparin, the suspicion of VITT or HIT prompted the administration of intravenous immunoglobulin (IVIG).
7 The platelet counts gradually increased to 104,000/µL after two days of IVIG infusion with the dose of 1g/kg of bodyweight (which in turn 60 g), and anticoagulation with apixaban at a reduced dose due to decreased renal function (Scr, 1.58 mg/dL) was initiated. Calf swelling was resolved, and the platelet count reached 145,000/µL 17 days after IVIG injection (
Fig. 1). Anti-heparin PF4 IgG by both enzyme-linked immunosorbent assay (ELISA, LIFECODES PF4 IgG [Immucor]) (2.54 OD; reference range, < 0.4 OD) and the chemiluminescent immunoassay (analysis machine: ACL-TOP 700 [Werfen]; Reagent, HemosiL HIT-Ab [HemosiL]) (43.60 U/mL; reference rage, < 1.00 U/mL) was confirmed to be positive. The patient was discharged home while receiving oral apixaban.
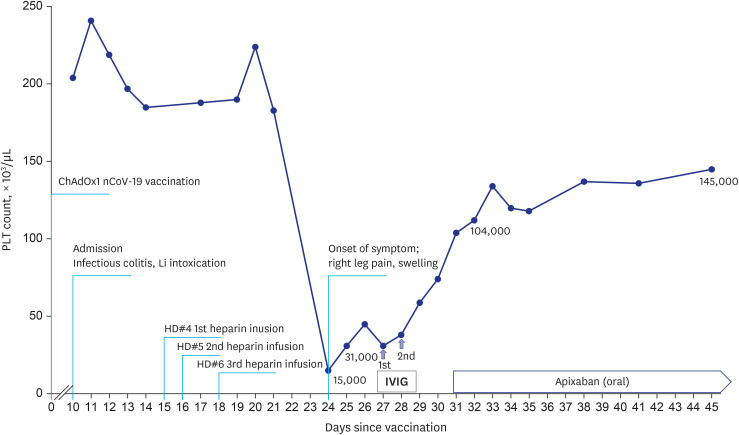 | Fig. 1
Serial PLT counts are presented in order to clinical events. A 70-year-old man who had thrombotic thrombocytopenia was complicated by deep vein thrombosis of lower extremity after vaccination. After administration of intravenous immunoglobulin with dose of 1 g/kg for 2 consecutive days, the PLT count gradually rose to 104,000/µL.
PLT = platelet, IVIG = intravenous immunoglobulin.

|
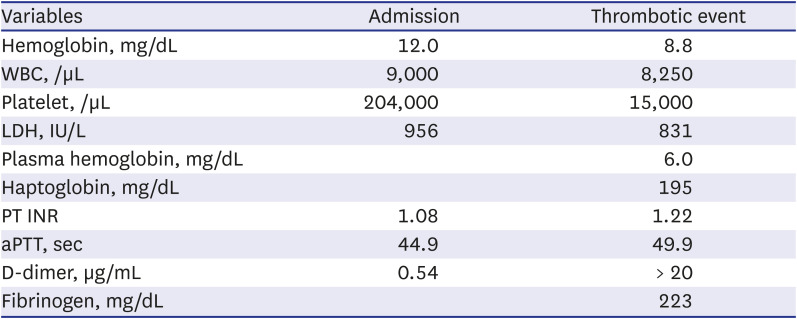
Table 1
Disseminated intravascular coagulation profile at admission and thrombotic event
|
Variables |
Admission |
Thrombotic event |
|
Hemoglobin, mg/dL |
12.0 |
8.8 |
|
WBC, /µL |
9,000 |
8,250 |
|
Platelet, /µL |
204,000 |
15,000 |
|
LDH, IU/L |
956 |
831 |
|
Plasma hemoglobin, mg/dL |
|
6.0 |
|
Haptoglobin, mg/dL |
|
195 |
|
PT INR |
1.08 |
1.22 |
|
aPTT, sec |
44.9 |
49.9 |
|
D-dimer, µg/mL |
0.54 |
> 20 |
|
Fibrinogen, mg/dL |
|
223 |







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download