1. O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013; 61(4):e78–140. PMID:
23256914.
2. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018; 39(2):119–177. PMID:
28886621.
3. Sim WJ, Ang AS, Tan MC, Xiang WW, Foo D, Loh KK, et al. Causes of delay in door-to-balloon time in south-east Asian patients undergoing primary percutaneous coronary intervention. PLoS One. 2017; 12(9):e0185186. PMID:
28934306.

4. Wong CK. Minimizing false activation of cath lab for STEMI--a realistic goal? Int J Cardiol. 2014; 172(1):e91–e93. PMID:
24444482.
5. Lopez-Jimenez F, Attia Z, Arruda-Olson AM, Carter R, Chareonthaitawee P, Jouni H, et al. Artificial intelligence in cardiology: present and future. Mayo Clin Proc. 2020; 95(5):1015–1039. PMID:
32370835.

6. Urtnasan E, Park JU, Joo EY, Lee KJ. Identification of sleep apnea severity based on deep learning from a short-term normal ECG. J Korean Med Sci. 2020; 35(47):e399. PMID:
33289367.

7. Park CW, Seo SW, Kang N, Ko B, Choi BW, Park CM, et al. Artificial intelligence in health care: current applications and issues. J Korean Med Sci. 2020; 35(42):e379. PMID:
33140591.

8. Erdenebayar U, Kim H, Park JU, Kang D, Lee KJ. Automatic prediction of atrial fibrillation based on convolutional neural network using a short-term normal electrocardiogram signal. J Korean Med Sci. 2019; 34(7):e64. PMID:
30804732.

9. Kim JK, Choo YJ, Choi GS, Shin H, Chang MC, Park D. Deep learning analysis to automatically detect the presence of penetration or aspiration in videofluoroscopic swallowing study. J Korean Med Sci. 2022; 37(6):e42. PMID:
35166079.

10. Kang M, Hong KS, Chikontwe P, Luna M, Jang JG, Park J, et al. Quantitative assessment of chest CT patterns in COVID-19 and bacterial pneumonia patients: a deep learning perspective. J Korean Med Sci. 2021; 36(5):e46. PMID:
33527788.

11. Hughes JW, Olgin JE, Avram R, Abreau SA, Sittler T, Radia K, et al. Performance of a convolutional neural network and explainability technique for 12-lead electrocardiogram interpretation. JAMA Cardiol. 2021; 6(11):1285–1295. PMID:
34347007.

12. Guo C, Pleiss G, Sun Y, Weinberger KQ. On calibration of modern neural networks. Proceedings of the 34th International Conference on Machine Learning, PMLR. 2017; 70:1321–1330.
13. Lin TY, Goyal P, Girshick R, He K, Dollár P. Focal loss for dense object detection. Proceedings of the IEEE International Conference on Computer Vision (ICCV). 2017. p. 2980–2988.

14. Park J, Choi KH, Lee JM, Kim HK, Hwang D, Rhee TM, et al. Prognostic implications of door-to-balloon time and onset-to-door time on mortality in patients with ST-segment-elevation myocardial infarction treated with primary percutaneous coronary intervention. J Am Heart Assoc. 2019; 8(9):e012188. PMID:
31041869.

15. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44(3):837–845. PMID:
3203132.

16. Moskowitz CS, Pepe MS. Comparing the predictive values of diagnostic tests: sample size and analysis for paired study designs. Clin Trials. 2006; 3(3):272–279. PMID:
16895044.

17. Altman DG. Practical Statistics for Medical Research. London, UK: Chapman & Hall;1990.
18. McNamara RL, Wang Y, Herrin J, Curtis JP, Bradley EH, Magid DJ, et al. Effect of door-to-balloon time on mortality in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2006; 47(11):2180–2186. PMID:
16750682.

19. Brodie BR, Hansen C, Stuckey TD, Richter S, Versteeg DS, Gupta N, et al. Door-to-balloon time with primary percutaneous coronary intervention for acute myocardial infarction impacts late cardiac mortality in high-risk patients and patients presenting early after the onset of symptoms. J Am Coll Cardiol. 2006; 47(2):289–295. PMID:
16412849.

20. Rathore SS, Curtis JP, Chen J, Wang Y, Nallamothu BK, Epstein AJ, et al. Association of door-to-balloon time and mortality in patients admitted to hospital with ST elevation myocardial infarction: national cohort study. BMJ. 2009; 338:b1807. PMID:
19454739.

21. Parikh R, Faillace R, Hamdan A, Adinaro D, Pruden J, DeBari V, et al. An emergency physician activated protocol, ‘Code STEMI’ reduces door-to-balloon time and length of stay of patients presenting with ST-segment elevation myocardial infarction. Int J Clin Pract. 2009; 63(3):398–406. PMID:
19222625.

22. Khot UN, Johnson ML, Ramsey C, Khot MB, Todd R, Shaikh SR, et al. Emergency department physician activation of the catheterization laboratory and immediate transfer to an immediately available catheterization laboratory reduce door-to-balloon time in ST-elevation myocardial infarction. Circulation. 2007; 116(1):67–76. PMID:
17562960.

23. Ibrahim F, Akhtar N, Salam A, Kamran S, Deleu D, D’Souza A, et al. Stroke Thrombolysis protocol shortens “Door-to-Needle Time” and improves outcomes-experience at a tertiary care center in qatar. J Stroke Cerebrovasc Dis. 2016; 25(8):2043–2046. PMID:
27256170.

24. Ido MS, Okosun IS, Bayakly R, Clarkson L, Lugtu J, Floyd S, et al. Door to intravenous tissue plasminogen activator time and hospital length of stay in acute ischemic stroke patients, Georgia, 2007-2013. J Stroke Cerebrovasc Dis. 2016; 25(4):866–871. PMID:
26853143.

25. Hwang YM, Kim JH, Kim YR. Comparison of mobile application-based ECG consultation by collective intelligence and ECG interpretation by conventional system in a tertiary-level hospital. Korean Circ J. 2021; 51(4):351–357. PMID:
33821585.

26. Santhanakrishnan R, Wang N, Larson MG, Magnani JW, Vasan RS, Wang TJ, et al. Racial differences in electrocardiographic characteristics and prognostic significance in Whites versus Asians. J Am Heart Assoc. 2016; 5(3):e002956. PMID:
27016575.

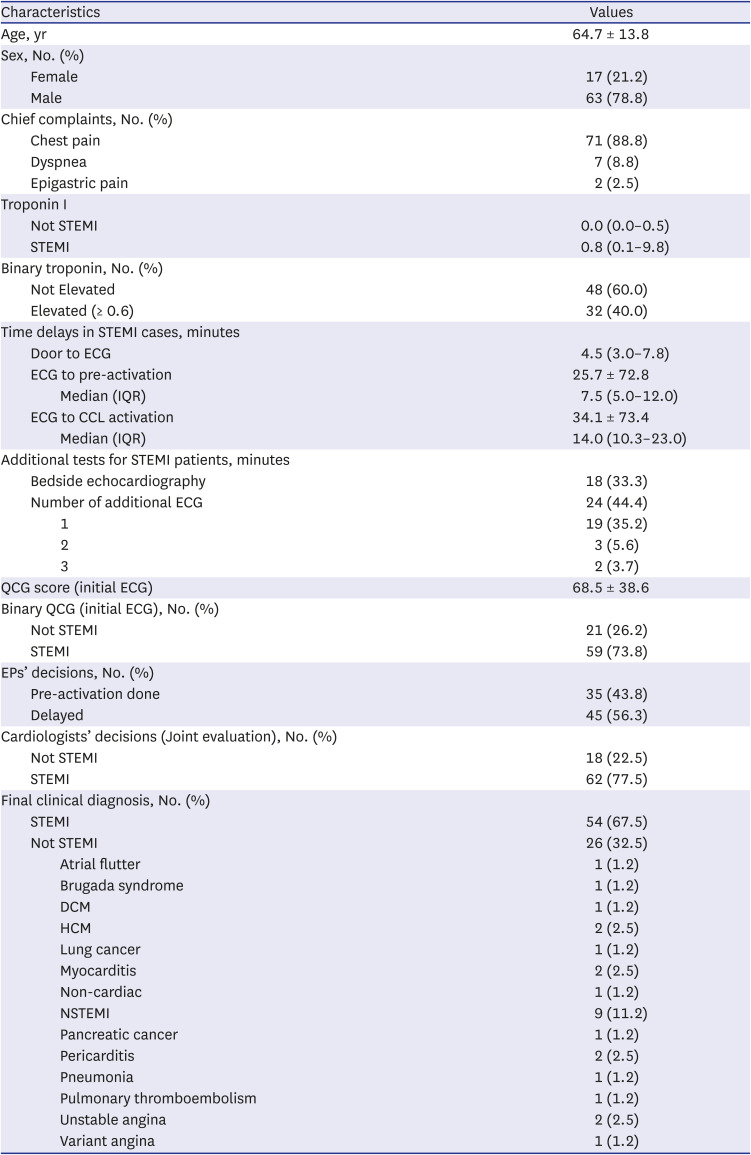
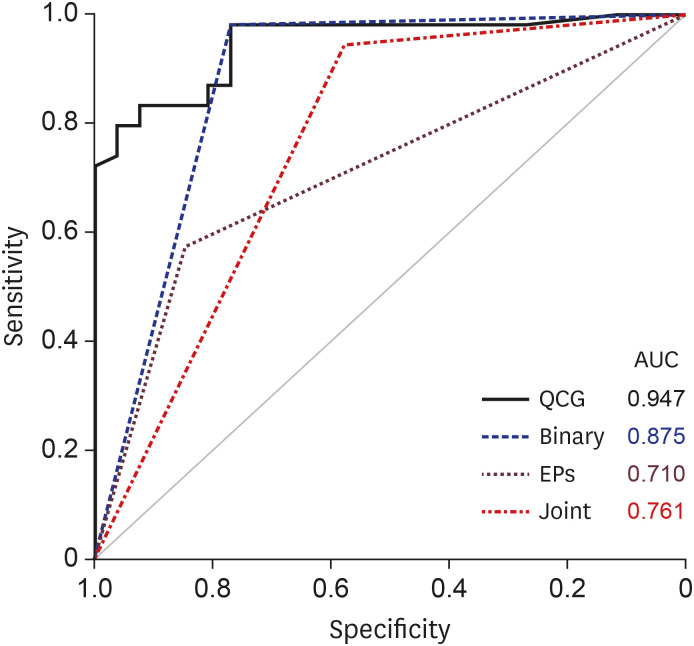
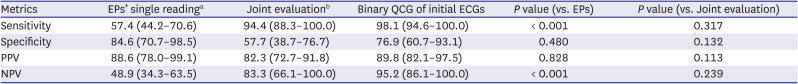

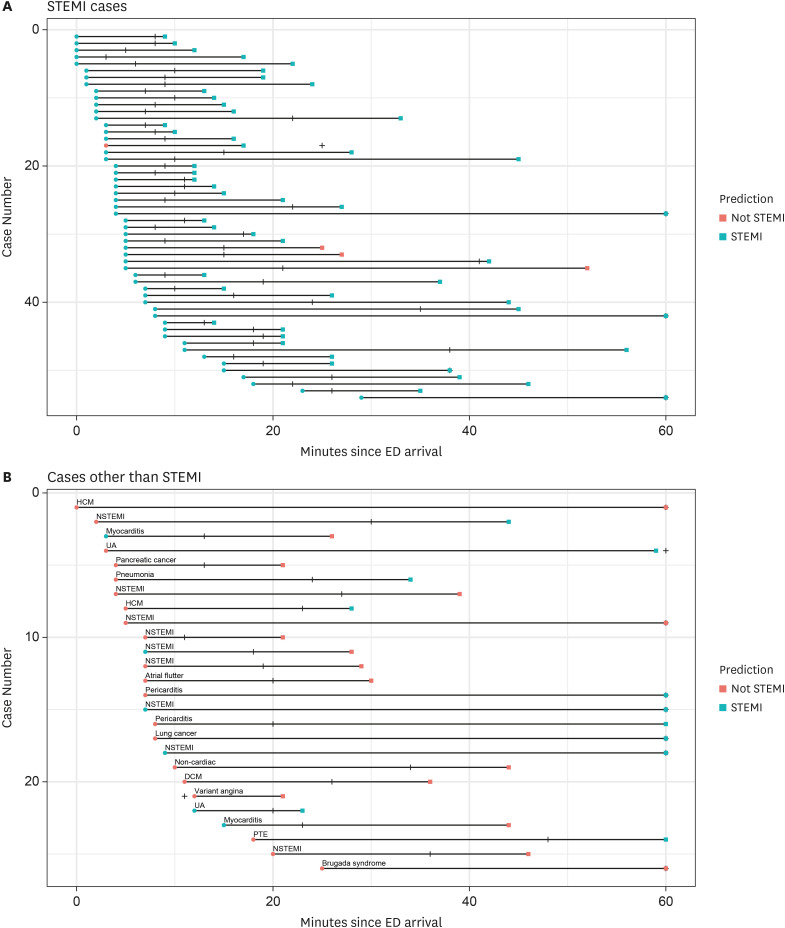




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download