INTRODUCTION
Vibrio vulnificus is a gram-negative, halophilic bacterium belonging to the genus
Vibrio and family
Vibrionaceae. It is commonly found in warm seawater and proliferates at a water temperature of ≥ 20ºC.
12
V. vulnificus is an important opportunistic pathogen that can induce primary septicemia through the consumption of contaminated raw or undercooked seafood or wound infection through the exposure of a wound to warm seawater inhabited by the organism.
234
V. vulnificus is naturally present in estuarine environments and coastal waters and is commonly found in oysters and molluscan shellfish.
56 It is a major pathogen that accounts for 95% of all seafood-related deaths in the United States and has the highest fatality rate among foodborne pathogens.
67
In patients with chronic liver disease, especially cirrhosis,
V. vulnificus infection is a highly lethal condition that usually manifests 1–2 days after exposure to the organism.
8 Although the infective or lethal dose of
V. vulnificus in humans is unknown, host susceptibility is an important factor in this infection.
9 Immunocompromised patients, including those with chronic liver diseases and cancer, have an increased risk of infection and complications.
4
V. vulnificus infection has been reported in some coastal cities in the United States, Japan, and Taiwan,
10 as well as in France, Demark, Israel, Germany, and Greece.
11
V. vulnificus infection is a serious public health concern in Korea, as it occurs annually in the summer and has a high case fatality rate of approximately 50%.
5 In Korea,
V. vulnificus infection has been monitored since its legal designation as a national notifiable disease on August 1, 2000, and reporting of confirmed or suspected cases is mandatory. However, although there have been some studies on the epidemiology of
V. vulnificus infection in Korea,
1213 many of its epidemiological characteristics remain unknown.
The present study was designed to analyze the incidence of all notified cases of V. vulnificus infection by time, place, and person from 2001 to 2016, to identify the epidemiologic features and to estimate the case fatality rate using a case epidemiological investigation report by the Korea Centers for Disease Control and Prevention (KCDC, currently Korea Disease Control and Prevention Agency).
METHODS
The scope of the mandatory reporting of
V. vulnificus infection, a legally designated infectious disease, covers confirmed or suspected infections. According to the surveillance manual for national notifiable diseases from the KCDC,
14 confirmed cases were defined as cases where patients showed clinical symptoms matching the definition of
V. vulnificus infection and confirmed by laboratory testing, whereas suspected cases were defined as cases where patients showed clinical symptoms matching the definition of
V. vulnificus infection with epidemiological association but were negative on laboratory tests. In this study, we included all reported cases of
V. vulnificus infection.
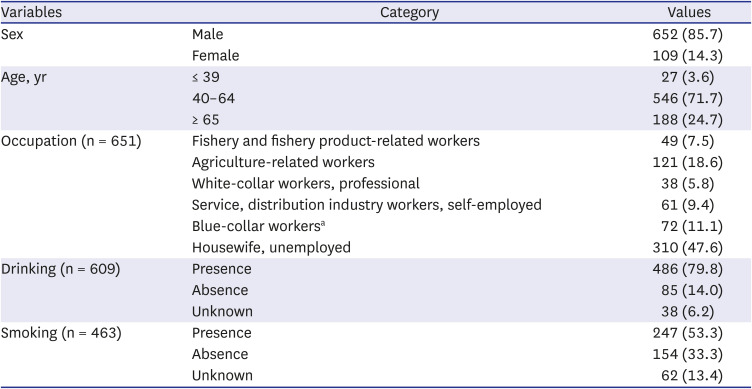
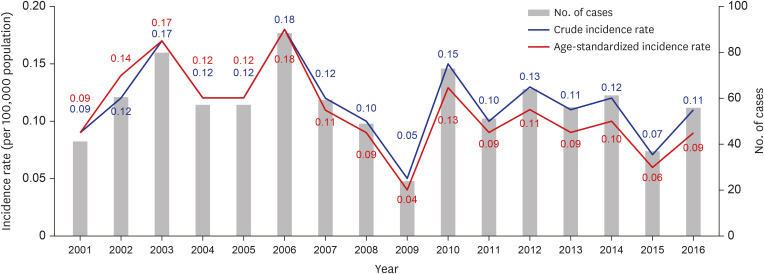
From 2001 to 2016, 913 cases of V. vulnificus infection were identified (835 confirmed and 78 suspected). We estimated the yearly, monthly, and regional incidence of all 913 cases.
In addition to the surveillance data reported to the KCDC, the number of patients who were under the National Health Insurance Service (NHIS) during the study period was collected by year, month, and region. Seawater temperature data for the study period were provided by the Korea Oceanographic Data Center of the National Institute of Fisheries Science.
The standard case report form of epidemiological investigation for V. vulnificus infection was launched in 2003, and a follow-up investigation protocol was launched in 2011 to determine the outcome of cases. We analyzed the standard epidemiological investigation forms of all 761 cases from 2003 to 2016 to determine the detailed demographic factors, clinical features, source of infection, and distribution of underlying diseases. The 152 reported cases who could not be followed up were excluded from the analysis. From all 761 notified cases (696 confirmed and 65 suspected) between 2003 and 2016, we identified the general characteristics (sex, age, occupation, and region of residence), clinical characteristics (date of onset, clinical symptoms, and signs), underlying diseases, source of infection (place of infection, history of seafood consumption, and history of seawater exposure), lifestyle (drinking and smoking status), and outcome (death or survival) of the cases.
Statistical analysis
The incidence rate was calculated as the newly notified number divided by 100,000, according to a specific year or region. The case fatality rate was calculated as the proportion of deaths (%) among all reported cases. Frequency analysis was performed to identify descriptive epidemiological features according to time, place, and personal characteristics. Frequency analysis was also used to compare the number of V. vulnificus infection cases reported to the KCDC with the number of patients under the NHIS.
Ethics statement
This study was approved by the Institutional Review Board of Korea University (KUIRB-2018-0025-01). Informed consent was waived because of the retrospective nature of the study.
DISCUSSION
V. vulnificus infection occurs annually in Korea with regional and seasonal features. In our analysis of the cases of
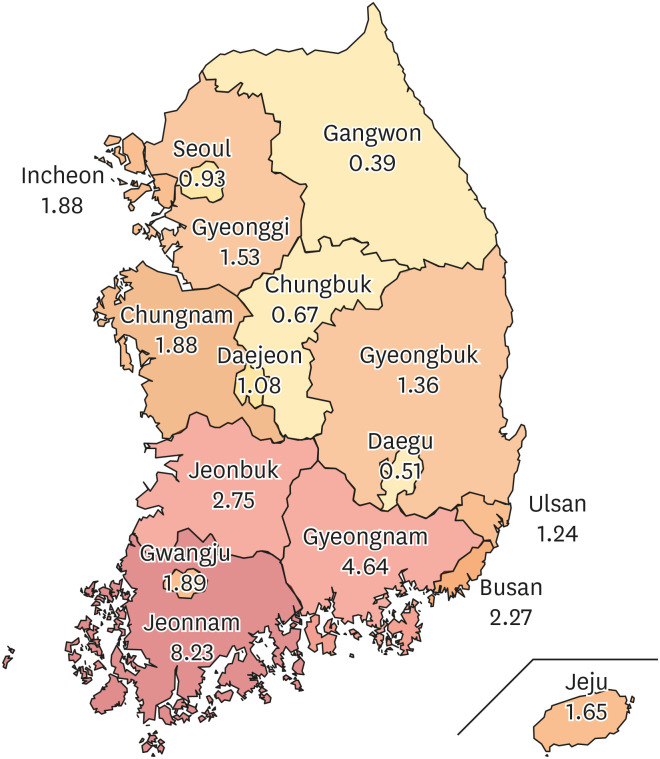
V. vulnificus infection from 2001 to 2016, the incidence rate was 0.12 ± 0.03 per 100,000 population, with the highest incidence (8.23) recorded in the Jeonnam region.
V. vulnificus infection mostly occurs sporadically in the United States. From 1998 to 2007, 276 cases of
V. vulnificus infection were reported in Florida, with an average annual incidence rate of 1.6 per 1 million.
15 In Japan, 12–24 cases of
V. vulnificus infection have been reported annually.
16 In Taiwan,
17 the estimated prevalence of
V. vulnificus infection from 1985 to 2000 was 0.35–1.24 per 1 million, with the largest number of cases reported in 2000 (n = 26).
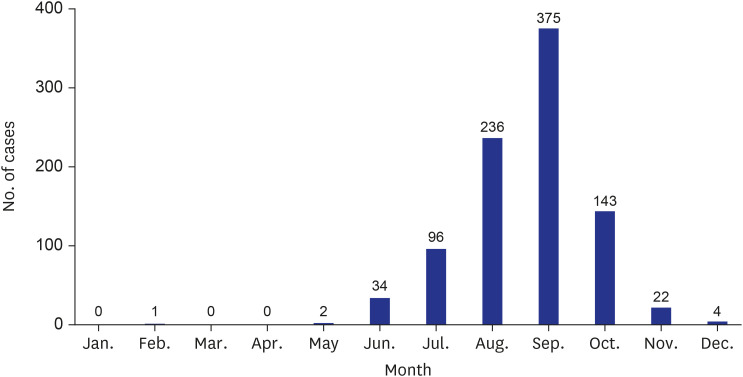
In our study, the largest number of cases occurred in September (41.1%), and 82.6% of all cases occurred between August and October. Similarly, most cases of infection in the United States were reported between April and October, which are the months during which the organism flourishes.
18 In Japan, 81.1% of the cases were reported between July and September,
19 with the greatest number reported in July.
16 In Taiwan, almost all cases occurred between late spring and early fall (April through October), a period during which the seawater temperature ranges from 20
°C to 29
°C.
17
In a previous study,
20
Vibrio spp. isolated from the environments of the southern coastal tidal water and mud samples of South Korea showed species-specific seasonal patterns, with
V. vulnificus limited to summer. Therefore, the results of this previous study
20 support the results of our study, which showed that
V. vulnificus infection cases occurred intensively in August and October, and the incidence rate per 100,000 population was the highest in Jeonnam (8.23).
It can be assumed that the warm seawater temperature affected the incidence. A previous study,
21 showed a positive relationship between seawater temperature and
V. vulnificus cases and the relative risks of
V. vulnificus infection in the south, west, and east coasts according to the 1°C increase in seawater temperature were 1.35 (95% confidence interval [CI], 1.19–1.53), 1.34 (95% CI, 1.20–1.51), and 1.30 (95% CI, 1.06–1.59), respectively.
According to a previous study on cases reported in Florida from 1981 to 1992,
22 oyster consumers with liver disease had 80 times higher risk of
V. vulnificus infection. In this study, the risk of
V. vulnificus infection was associated with male sex (85.7%), older age (96.5% > 40 years), and underlying disease (96.1%). Similar demographic characteristics have been reported in the United States,
23 Taiwan,
17 and Japan.
19 Leng et al.
10 reported that the higher infection rate among men (86.1%) is attributable to higher alcohol use and a higher percentage of occupational exposure to seawater and seafood.
24 In our study, 96.1% of the cases had an underlying disease, with liver cirrhosis (56.3%) being the most common, followed by diabetes mellitus (26.7%). In a study that investigated 37 cases in the Ariake Sea region in Japan between 1984 and 2008, 91.6% of the patients had liver disease.
19 In a Taiwanese study that investigated 84 patients between 1995 and 2000, over 80% of the patients had liver disease, mainly caused by hepatitis B or C infection, followed by diabetes and steroid use.
17 The most common comorbidities among patients with
V. vulnificus infection in the United States were heart disease (34%), diabetes mellitus (23%), alcohol consumption (22%), and liver disease (20%).
23
In Korea, seafood consumption is the most common (90.1%) route of
V. vulnificus infection, resulting in primary sepsis. In a Japanese study, 31 of 37 (83.8%) patients consumed raw seafood, and the remaining six patients had an unknown route of infection, with no clear cases of infection through wound sites.
19
In our study, 81.2% of the patients with an underlying disease had liver disease (liver cirrhosis, hepatitis, or liver cancer). These patients most frequently contracted the pathogen through seafood consumption, whereas patients without liver disease most frequently contracted the pathogen through seawater exposure, with the difference being statistically significant (
P = 0.035). As liver disease is a potent predictor of death in all patients, a dysfunctional liver is reported to impose an additional risk through an independent mechanism of transferrin saturation or iron overload.
24 Because of the strong correlation between liver disease and death, previous studies recommended that patients with liver disease should refrain from activities that involve seawater exposure if they currently have a wound or are likely to sustain a wound injury.
2223
In our study, 159 of 325 patients with
V. vulnificus infection died (48.9%). In a Taiwanese study of 84 patients with
V. vulnificus infection between 1995 and 2000, 25 patients died (29.8%), 57 patients survived (67.9%), and two patients had an unknown status (2.3%).
17 In a study in Japan, 24 of 37 patients died (64.9%).
19 Yun et al.
12 reviewed the clinical data of 34 patients in a Korean hospital between 2000 and 2011, among whom 16 patients (47.1%) died.
In the USA and Korea,
V. vulnificus infection is a nationally notifiable disease, but there is no obligation to report it in Japan.
16 The Centers for Disease Control and Prevention in the USA monitors vibriosis through the nationwide Cholera and Other
Vibrio Illness Surveillance (COVIS) system since 1988 and the 10-state Foodborne Diseases Active Surveillance Network (FoodNet) since 1996.
25 COVIS is a passive surveillance system to which all states can report laboratory-confirmed
Vibrio infections, and FoodNet conducts an active, population-based surveillance in ten states for all laboratory-confirmed
Vibrio infections, as well as other enteric infections transmitted commonly through food.
25 The three commonly reported
Vibrio species were
V. parahaemolyticus,
V. vulnificus, and
V. alginolyticus, and in both systems, most hospitalizations and deaths were caused by
V. vulnificus infection.
25 In Korea, since 2000, a passive report-based national notifiable infectious disease surveillance system has been operated to notify suspected and confirmed cases of
V. vulnificus infection. Since 2002, laboratory surveillance for pathogenic
Vibrio (vibrioNet) has been conducted to monitor the distribution status of three pathogenic
Vibrio species (
V. vulnificus,
V. cholerae, and
V. parahaemolyticus) and changes in marine environmental factors (seawater temperature, salinity, pH, turbidity).
From the seawater temperature analysis provided by KCDC from 2001 to 2016, the seawater temperature in August was the highest at 24.1°C. However, in September, when the seawater temperature was 23.1°C, the number of cases was the highest at 375. During the same period, 850 (93.1%) of 913 notified cases occurred between July and October, and the average seawater temperature between July and October was 22.1°C. Therefore, the occurrence of V. vulnificus infection in our study showed a distinct seasonality due to seawater temperature, and it occurred more often in people with underlying diseases such as liver cirrhosis; therefore, host factors can also affect the occurrence of disease.
V. vulnificus infection surveillance results showed a monthly average of 57
V. vulnificus infection cases, but given the passive reporting system and the number of cases where patients died without treatment or diagnosis, it has been reported that the actual number of cases could be much higher than this.
26 From 1981 to 1987, among
V. vulnificus patients reported in Florida, USA, the case fatality rates of those with and without liver disease were similar at 52% and 62%, respectively.
27 In our study, the case fatality rates of
V. vulnificus infection cases with and without liver disease were 51.2% and 42.5%, respectively, and there was no statistically significant difference (χ
2 = 1.65,
P = 0.199). In the future, a retrospective study is needed to identify possible risk factors that may affect mortality by analyzing the medical records of cases.
Our study analyzed all notified cases from investigation reports between 2003 and 2016 in Korea; however, we could not include data on patients’ blood tests, antibiotic use, and specific treatment in our analysis because of the lack of such information in epidemiological reports.
In conclusion, considering the high fatality rate associated with
V. vulnificus, it is important to establish public health measures to prevent
V. vulnificus infection. To lower the incidence of, and death from,
V. vulnificus infection, it is important to increase awareness among people at high risk, especially those living in high-risk areas. The public health approach should emphasize the prevention of
V. vulnificus infection.
6 Moreover, important approaches for reducing the disease burden include education and prevention strategies.
24 The Taiwanese government requires food companies to provide warning labels on seafood containers, menus, and public health brochures.
17 Therefore, additional research needs to be conducted in the future.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download