Shortly after the first case of the omicron variant (BA.1 sub-lineage of B.1.1.529) of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) was confirmed in South Africa on November 21, 2021, the World Health Organization documented its spread across the globe.
1 Along with the wide and rapid spread of the omicron variant, substantial mutations in its spike protein have raised new concerns about its increased transmissibility and ability to reduce vaccine effectiveness.
2 Accumulating evidence supports the high transmissibility and immune-evasion capability of the omicron variant
34 as well as a significant decrease in vaccine efficacy.
5
In South Korea, a booster dose of the coronavirus disease 2019 (COVID-19) vaccine was rolled out in the third week of November 2021. The South Korean government encouraged individuals over 60 years of age to receive a booster COVID-19 mRNA vaccine, achieving a vaccination rate of 90% in the elderly by December 2, 2021.
6 However, increased infection by the omicron variant and its capability to evade vaccine protection has become an imminent threat in South Korea.
This study aimed to compare the neutralization activity of the booster vaccine against the omicron variant with that against the wild-type and delta variants in South Korea.
Healthy elderly (≥ 75 years) who were scheduled to receive the booster vaccine were recruited for the study beginning the first week of November 2021. Serum samples for another study, which were obtained from young (< 60 years) healthy healthcare workers who had completed both full primary and booster vaccination, were used for the analysis as well. A history of SARS-CoV-2 infection or exposure was screened at each visit for the sampling. Two samples of serum were obtained from 15 young healthcare workers and 15 elderly individuals after receiving the full primary dose of the BNT162b2 vaccine (baseline) and then again 4 weeks after the booster vaccine.
The plaque reduction neutralization test was employed to measure the 50% neutralizing dilution (ND
50) titers against the wild-type (BetaCoV/Korea/KCDC03/2020, NCCP43326), delta (hCoV-19/Korea/KDCA119861/2021, NCCP43390), and omicron (B.1.1.529) variants in serum samples. The omicron variant was isolated from nasopharyngeal swabs of a patient who was screened and confirmed by the Korea Disease Control and Prevention Agency. Serial dilution endpoint neutralization testing was performed in a BL3 facility. Five-fold serial dilutions (1:5 to 1:15,625) of heat-inactivated (56°C, 30 minutes) serum samples were pre-incubated with 50 plaque-forming units of SARS-CoV-2 virus in cell-free plates for 1 hour at 37°C. After neutralization, 2.0 × 10
5/mL VeroE6 cells (KCTC, AC28803) were inoculated with diluted virus-serum solutions in 12-well plates. The plates were incubated at 37°C under a 5% CO
2 atmosphere for 3–5 days. Subsequently, cells were fixed in 7% formaldehyde and stained with crystal violet. The Spearman–Kärber method was used to calculate ND
50 titer values.
7
Tests were independently performed twice on each sample. Statistical analysis was conducted using IBM SPSS Statistics v26 software (IBM Corp., Armonk, NY, USA). Continuous variables were analyzed using the Student’s t-test, Mann-Whitney U test, and Kruskal-Wallis test. The Chi-square test was used for the analysis of categorical variables. Statistical significance was considered at P < 0.05.
The median age of the study participants was 67 years and the male-to-female ratio was 13:17. The median ages of the older and younger groups were 81 and 35 years, respectively. The median time from the second vaccine dose to baseline testing was 182 days (
Table 1), with a median interval of 6.3 and 5.9 months for the older and younger groups, respectively. The median interval from booster vaccination to testing was 25 and 27 days for the older and younger groups, respectively. No participant reported a history of SARS-CoV-2 infection or exposure.
Table 1
Characteristics of the study population
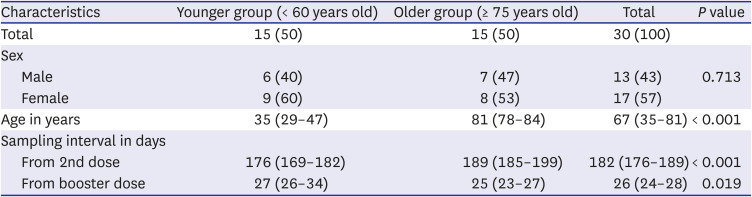
|
Characteristics |
Younger group (< 60 years old) |
Older group (≥ 75 years old) |
Total |
P value |
|
Total |
15 (50) |
15 (50) |
30 (100) |
|
|
Sex |
|
|
|
|
|
Male |
6 (40) |
7 (47) |
13 (43) |
0.713 |
|
Female |
9 (60) |
8 (53) |
17 (57) |
|
|
Age in years |
35 (29–47) |
81 (78–84) |
67 (35–81) |
< 0.001 |
|
Sampling interval in days |
|
|
|
|
|
From 2nd dose |
176 (169–182) |
189 (185–199) |
182 (176–189) |
< 0.001 |
|
From booster dose |
27 (26–34) |
25 (23–27) |
26 (24–28) |
0.019 |

The overall median ND
50 titers against BetaCoV/Korea/KCDC03/2020, delta, and omicron at baseline were 144.7, 24.2, and 5.3, respectively (
Fig. 1). In the older group, the median ND
50 titers against BetaCoV/Korea/KCDC03/2020, delta, and omicron were 63.5, 16.0, and < 5, compared to 237.1, 68.1, and 6.9 in the younger group, respectively (
Fig. 1). The ND
50 titers against BetaCoV/Korea/KCDC03 and delta in the older group at baseline were significantly lower than those in the younger group (
P = 0.001 and
P = 0.005, respectively). However, the ND
50 titers against omicron did not differ significantly between the two groups at baseline (
P = 0.182).
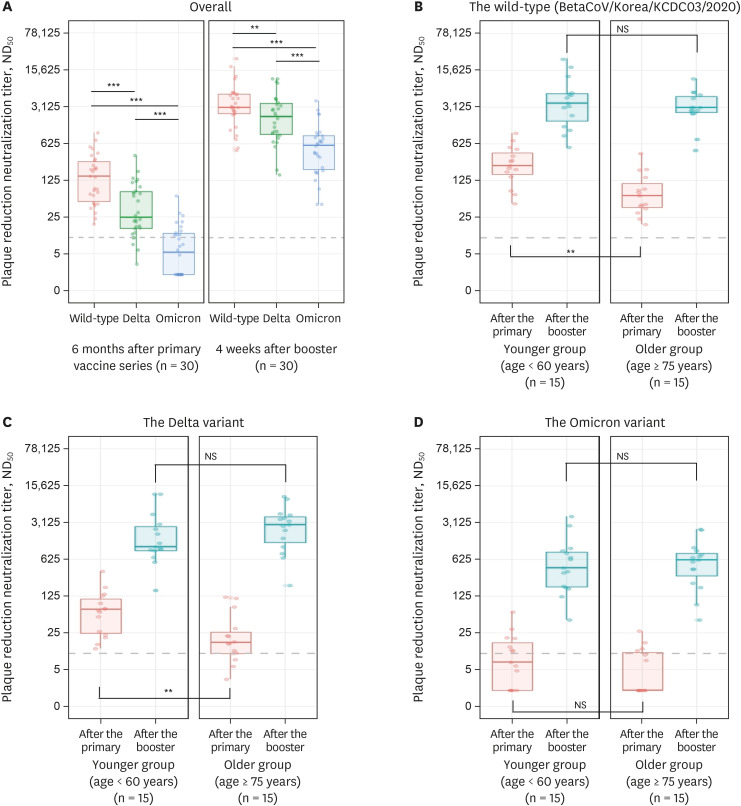 | Fig. 1
Neutralizing antibody titers against variants of SARS-CoV-2: Wild-type (BetaCoV/Korea/KCDC03/2020), delta, and omicron. The panels display the median ND50 values (A) overall and by age group against the (B) wild-type (BetaCoV/Korea/KCDC03/2020), (C) delta, and (D) omicron variants (younger group, < 60 years old; older group, ≥ 75 years old). Antibody titers ≤ 10 was considered below the detection threshold (broken line). Boxes in each panel span the interquartile range; the solid line in each box indicates the median while the vertical line denotes the 2.5 and 97.5 percentile values. Comparisons between groups were analyzed by Student’s t-test and Mann-Whitney U test.
ND50 = 50% neutralization dilution, ns = not significant.
Statistical significance of differences between groups are defined as: ns
P > 0.05; **P < 0.01; ***P < 0.001.

|
Four weeks after receiving the booster vaccine, the overall median ND
50 titers increased approximately 21-, 84-, and 109-fold against BetaCoV/Korea/KCDC03/2020 (3,040.6), delta (2,010.1), and omicron (578.7), respectively (
Fig. 1). The median ND
50 titers against delta were significantly lower than those against BetaCoV/Korea/KCDC03/2020 (
P < 0.001), and those against omicron were significantly lower than those against delta (
P < 0.001). In the older group, the median ND
50 titers increased approximately 47-, 174-, and 120-fold against BetaCoV/Korea/KCDC03/2020 (3,012.8), delta (2,785.6), and omicron (601.4), respectively. Comparatively, the median ND
50 titers in the younger group against BetaCoV/Korea/KCDC03/2020, delta, and omicron increased approximately 15-fold (3,617.4), 15-fold (1,074.6), and 62-fold (427.6), respectively. The median ND
50 titers against BetaCoV/Korea/KCDC03/2020, delta, and omicron did not differ significantly between the two groups (
P = 0.520, 0.229, and 0.740, respectively).
Although infection with the omicron variant has caused less severe illness in South Africa,
8 the higher rate of transmission may cause a devastating burden to South Korea, regardless of the population’s higher vaccination rate.
9 Research suggests that protection against infection and mild symptomatic disease at 15 weeks after two doses of the BNT162b2 vaccine could be less than 50% for the omicron variant, compared to 63.5% for the delta variant.
10 However, the impact of the omicron variant on society may differ depending on the level of pre-existing population immunity and immune evasion properties of omicron.
4
In the current study, neutralizing antibody titers against BetaCoV/Korea/KCDC03/2020 were preserved 6 months after full primary BNT162b2 vaccination, whereas those against omicron were below the detectable level and lower than those against delta. A lower neutralizing antibody titer is known to be associated with a higher risk of symptomatic COVID-19.
11 Thus, limited neutralizing activity against the omicron variant will likely lead to breakthrough infections in individuals who have completed the full primary vaccine series.
Importantly, the booster BNT162b2 vaccine elicited substantially high serum neutralization activity against omicron. Nevertheless, the neutralizing antibody titers against omicron remained lower than those against BetaCoV/Korea/KCDC03/2020 and delta, which concurred with the results of previous studies.
1213 Our data indicate that the omicron variant has partial resistance to the BNT162b2 vaccine even though neutralizing antibody titers were improved after the booster dose. Hoffmann et al.
2 reported that a booster dose of the BNT162b2 vaccine increased the efficiency of inhibiting entry by the omicron spike protein compared to full primary vaccination. Taken together, these findings suggest that 2-dose immunization with BNT162b2 might not adequately protect against omicron infection, and a variant-specific vaccine might be needed for efficient protection against this variant.
214 However, vaccine effectiveness is determined not only by neutralizing antibodies but also by binding antibodies and T-cells, which play an important role in preventing severe disease.
15 Therefore, future data may reveal that preserving adaptive immunity other than neutralizing antibodies may prevent the risk of severe disease or death due to omicron infection in individuals who did not receive the booster vaccine.
16 Consequently, further follow-up is needed to determine protection against severe illness before and after booster vaccination.
In the current study, the younger group displayed higher neutralizing antibody titers than the detection threshold against BetaCoV/Korea/KCDC03/2020 and delta after the second vaccine dose. Moreover, titers were also higher than the detection threshold against BetaCoV/Korea/KCDC03/2020 in the older group. However, 20% (3/15) and 73.3% (11/15) of the older group displayed undetectable titers against delta and omicron, respectively, and 60.0% (9/15) of the younger group displayed undetectable titers against omicron. This result is concordant with a previous study in which 31.3% of elderly participants had undetectable levels of neutralizing antibodies even on the seventeenth day after the second vaccine dose.
17 Nevertheless, our results demonstrated that neutralizing antibody titers against omicron increased after booster vaccination regardless of age, suggesting that a booster vaccine may effectively protect both young and elderly adults against omicron.
1819
Despite the limitation of the small sample size, our study reports the earliest data suggesting increased activity of neutralizing antibodies after a booster BNT162b2 vaccine, which may induce robust neutralization and overcome immune evasion of the omicron variant. Furthermore, our results support a booster vaccination dose and prioritization of the vaccine program in South Korea.
Ethics statement
The study was approved by the institutional review board of the National Medical Center (NMC-2021-10-124 and NMC-2021-03-027) and performed in accordance with the ethical standards of the Declaration of Helsinki and its later amendments. Informed consent was obtained from all participants.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download