INTRODUCTION
After the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak, coronavirus disease 2019 (COVID-19) vaccines were developed at an unprecedented rate, and governments have been granting emergency use authority for these vaccines. In South Korea, vaccination was initiated in February 2021, starting with high-risk patients and first-line healthcare workers and gradually expanding to the general public; vaccination for 12
th graders was completed in August 2021. Age restrictions were lowered in October, allowing children over the age of 12 years to receive the vaccine.
1
However, questions regarding the safety of these COVID-19 vaccines due to a lack of clinical trials have been raised continuously. Several studies have reported rare but severe adverse reactions to COVID-19 mRNA vaccines.
23 Some studies have reported the possibility of a causal relationship between COVID-19 mRNA vaccination and the risk of myocarditis/pericarditis in adolescent and young adult males.
456 In clinical practice, as COVID-19 mRNA vaccination was expanded to the general population, the number of patients presenting to the ED with cardiovascular symptoms increased. Using mobile self-report questionnaires, a survey studies on adverse reactions following administration of the ChAdOx1 nCoV-19 vaccine and BNT162b2 vaccine in healthcare workers found that females and younger age groups reported adverse reactions more frequently than males and older age groups.
78 As such, there may be differences in adverse reactions by sex and age group.
Unlike general adverse reactions, such as fever, chills, myalgia, fatigue, and headache, cardiovascular symptoms require more attention from emergency physicians and more in-depth evaluation for a differential diagnosis considering other serious diseases, as these symptoms could be red flags for potentially life-threatening conditions. This has led to a substantial burden on first-line health care workers in the ED who are already occupied with COVID-19 screening tasks.
Therefore, we investigated the clinical characteristics of patients presenting to the ED with cardiovascular adverse reactions after COVID-19 mRNA vaccination and analyzed differences in clinical features according to sex and age group.
Go to :

METHODS
Study design and data collection
The ‘Infectious Disease Control and Prevention Act’ of South Korea requires medical staff to report any suspected COVID-19 vaccine-related adverse events. In compliance with this Act, we have been reporting adverse events to the COVID-19 Immunization Registry System (IRS) since March 2021.
We conducted a retrospective observational study in two hospitals, Incheon and Daejeon, whose EDs serve as regional emergency centers in South Korea. Among the patients who visited the EDs of the two hospitals between June 1, 2021, and October 15, 2021, patients with adverse reactions reported to the COVID-19 IRS were selected. The subjects for this study were included based on cardiovascular adverse reactions after COVID-19 mRNA vaccination and were 65 years or younger. The exclusion criteria were as follows: received of a viral vector COVID-19 vaccine, presented with noncardiovascular symptoms, aged more than 65 years, and with incomplete data. The types of COVID-19 mRNA vaccines included Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273). Chest pain/discomfort, dyspnea and palpitation were considered cardiovascular symptoms. Onset day was set to 0 days, if cardiovascular symptoms occurred on the day of vaccination.
We collected data on patient sex, age, type of vaccine administered, vaccine dose (first or second), chief complaints, vital signs upon admission, laboratory data, outcomes (discharge or admission), ED length of stay, intravenous hydration and parenteral medication prescribed during ED stay, and final diagnosis.
Statistical analysis
Data were analyzed as absolute frequencies, percentages, mean averages, and standard deviations. Age groups were divided into one of the following: 17 to 19 years, 20 to 29 years, 30 to 39 years, 40 to 49 years, 50 to 59 years, and 60 to 65 years.
We compared the mean of continuous variables using student’s t-test and compared the distribution of dichotomous variables using the chi-square test with Yates correction for continuity or Fisher’s exact test between male and female groups. A P value of 0.05 or less was considered a statistically significant difference. All statistical analyses were analyzed using R statistics version 4.1.1 (R foundation for statistical computing, Vienna, Austria).
Ethics statement
Institutional Review Board of Daejeon St. Mary’s Hospital and Incheon St. Mary’s Hospital approved this study (approval No. DC21RIDI0073 by Daejeon and OC21RIDI0122 by Incheon). Informed consent was waived because of the retrospective nature of this study.
Go to :

DISCUSSION
Large-scale public COVID-19 vaccination campaigns have led to an increasing number of patients presenting to the ED with vaccine-related adverse reactions. In a mobile-based study conducted in South Korea, the most common adverse reactions following BNT162b2 mRNA vaccinations (Pfizer-BioNTech) were muscle aches (69.1%), fatigue (65.7%), headache (48.7%), chills (44.2%), and fever (32.1%), while 13.5% reported chest discomfort and palpitation.
8 The Korea Disease Control and Prevention Agency reported that cardiovascular symptoms accounted for 13.6% of all reported COVID-19 mRNA-related adverse reactions.
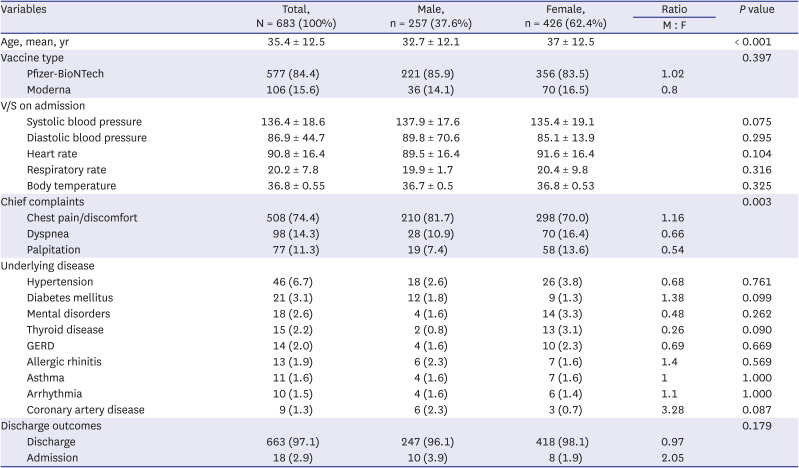
9 However, in this study, 712 (51%) out of 1,397 patients who visited the ED after receiving the COVID-19 mRNA vaccine reported cardiovascular symptoms as adverse reactions. This higher rate is thought to be because this study was conducted in an ED population.
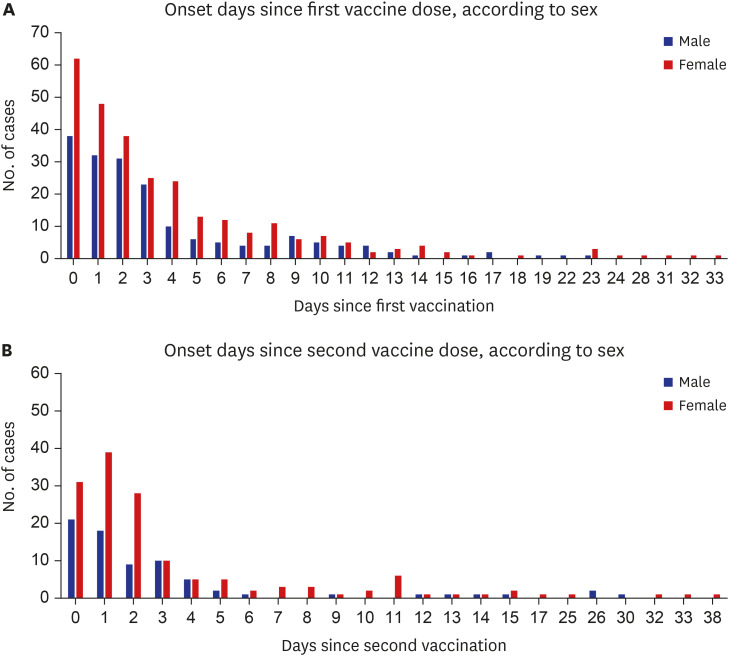
Myocarditis has been recognized as a rare complication of COVID-19 mRNA vaccination and usually occurs 2 to 3 days after receiving the second vaccine dose. The highest incidence was among young adult and adolescent males, and most cases of myocarditis were mild or moderate in severity.
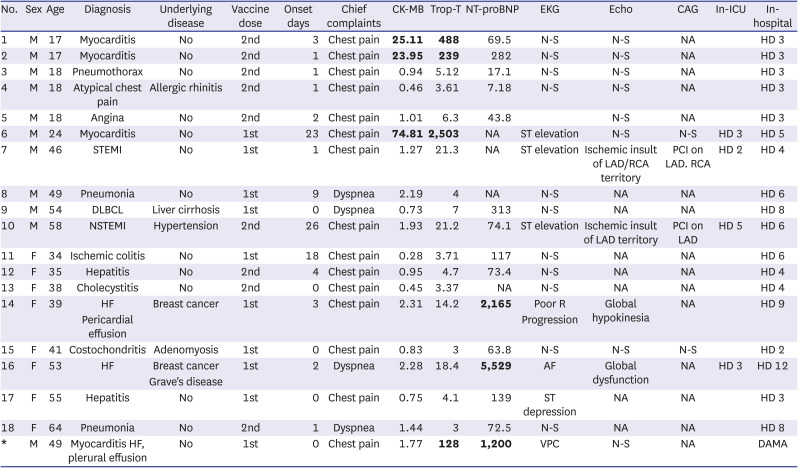
3510 In this study, four males were diagnosed with myocarditis. They were 17, 17, 24 and 49 years old, respectively; had elevated cardiac enzyme levels, nonspecific EKG findings (except for the 24-year-old patient) and no abnormalities on echocardiography; two out of the four patients developed symptoms after the second dose; and symptom onset was 0, 1, 3, and 23 days after vaccination, respectively. One study investigated adverse reactions until 21 and 30 days following the first and second doses, respectively.
3 Another study analyzed adverse reactions up to 42 days after the first dose.
11 In this study, the patient who developed symptoms on the 23
rd day after vaccination had ST elevation on his EKG, even though normal echocardiographic findings were observed. He underwent coronary angiography, which confirmed normal findings. In this patient with late onset, COVID-19 vaccination could not be excluded as a cause of myocarditis. All four cases of myocarditis had mild clinical progression, so cardiac magnetic resonance imaging (MRI) was not needed to confirm myocarditis. Myocarditis must be considered, especially in young adults and adolescents presenting to the ED with cardiovascular symptoms following COVID-19 mRNA vaccination, and invasive coronary angiography must be carefully considered.
Myocarditis/pericarditis is a rare disease commonly caused by viral infection, including SARS-CoV-2 infection.
12 The exact incidence rate of myocarditis is unknown due to difficulty in diagnosis.
121314 Endomyocardial biopsy (EMB) is the gold standard for a definitive diagnosis of myocarditis but is rarely performed due to the high-risk nature of the procedure. Recent advancements in cardiac MRI technology are associated with the increased annual incidence of myocarditis.
1516 The Center for Disease Control and Prevention (CDC) established a myocarditis case definition by adding cardiac MRI findings to Sagar's classification system.
17 Following the CDC definition, four cases of myocarditis in this study were classified as probable myocarditis, as the patients did not undergo EMB or cardiac MRI.
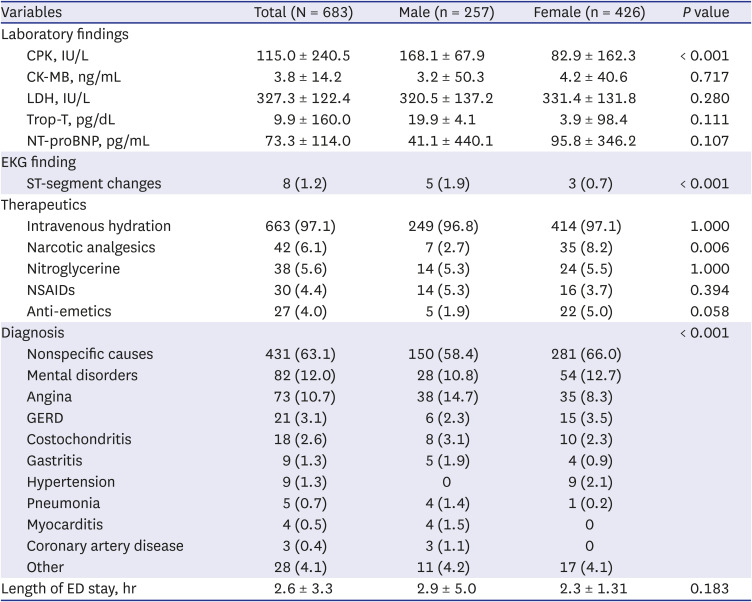
The final diagnoses included mental disorders (panic disorder, anxiety, or hyperventilation), GERD, costochondritis, and gastritis. These diseases can manifest as symptoms similar to cardiovascular symptoms, so a differential diagnosis is required. Additionally, two admitted males who visited the ED for vaccine adverse reactions were ultimately diagnosed with myocardial infarction. This suggests that emergency physicians must consider medical diseases other than adverse vaccine reactions.
In this study, prescriptions for nitroglycerine and analgesics were low. Considering that there were many nonspecific causes in the final diagnosis, it is thought that most patients had pain/discomfort that did not require pain control, although this is a limited interpretation of retrospective studies. In females, more narcotic medications were administered for pain relief than nonsteroidal anti-inflammatory drugs. This requires further research, utilizing a tool such as a pain score, in the future.
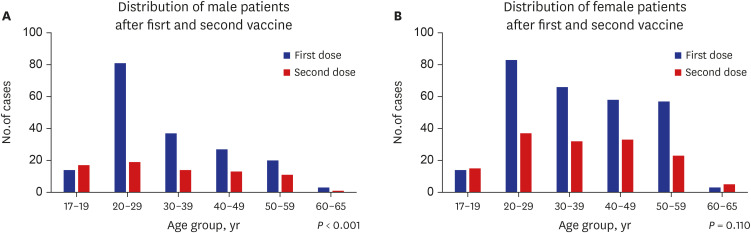
The 17–19 age group displayed different characteristics compared to the other age groups. They showed that there were more males than females, visited the ED more frequently after the second dose than after first dose, and higher diagnostic rates of myocarditis. These results were similar to those of other studies of myocarditis after COVID-19 mRNA vaccination.
31011
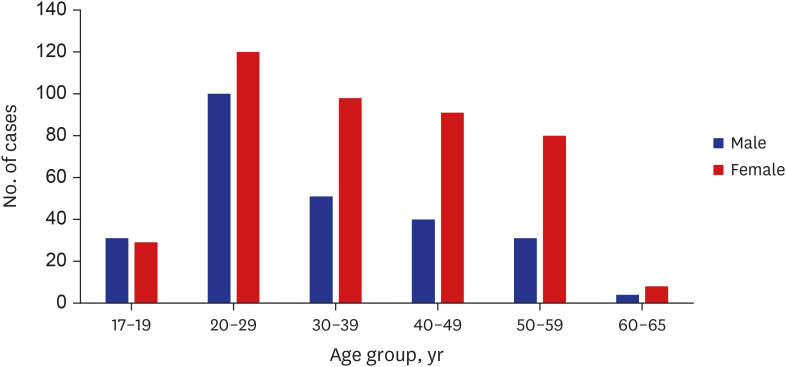
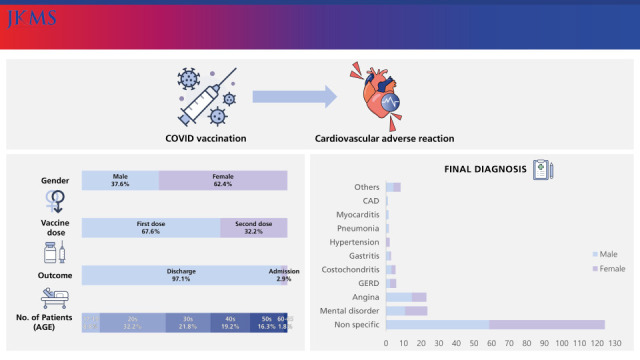
There were more females than males in this study, similar to other studies on vaccine adverse reactions.
78 One study reported that female sex was the main covariable, although a clear explanation of such an observation has not been provided.
18
Limitations in this study are its retrospective nature and small sample size. This study was performed in two hospitals; therefore, selection bias might have occurred. In addition, only patients between 17–19 years old were included in this study due to government age restrictions for vaccination; therefore, the results do not represent all teenagers. The results of vaccine types should be interpreted with caution, as the vaccination rates and administered vaccine types were different for each age group during this study period.
There are no studies on patients presenting to the ED with cardiovascular adverse reactions after COVID-19 vaccination. Therefore, this study can provide a guide for decision-making and patient disposition within the ED. In addition, this study can serve as a valuable reference for the comparison of adolescents and adults, following the South Korean government's decision to expand vaccinations to those 12 years of age or older.
In conclusion, the majority of patients with cardiovascular adverse reactions after COVID-19 mRNA vaccination were female, and visited the ED more frequently after the first vaccine dose. The number of patients in their 20s was the highest; most of patients were discharged from the ED; the admission rate was higher in males; and all four cases of myocarditis were diagnosed in males and showed mild clinical progression.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download