Accurate fluid management is a prime concern in perioperative patient care. The amount of fluid administration should be optimized based on the type of surgery or operative risk
12 because excessive intraoperative fluid volume can result in edema and organ dysfunction, while inadequate intraoperative fluid volume can result in hypoperfusion and organ ischemia.
3
Intravenous infusion flow regulators (IIFRs) are widely used to regulate fluid administration.
4 Because the numeric scales are marked on IIFRs, health care workers believe that they can precisely control the flow rate by scales. However, since the scales are marked based on the flow rate of normal saline, the accuracy is not known when using other fluids. There are many factors that might interfere with the accuracy of IIFR scales, and there is no accredited institution that verifies the accuracy of them.
As shown in Poiseuille’s Law, Q = {(πPr) ^ 4}/8 μL, the flow rate of the fluid (Q) is determined by the viscosity of the fluid (μ). By experience, the viscosity of each type of fluid is considered different from each other, but there is no data of flow rate regarding the exact viscosity of crystalloids and colloids. If the preset infusion rate differs from the actual infusion rate due to high viscosity, the difference could lead to overhydration or underhydration and harm the patient. Thus, in this study, the effects of intravenous (IV) fluid viscosity on the infusion flow rate when using IIFRs were assessed. The primary outcome was that the actually measured flow rate of fluid with various viscosities were different from the preset infusion rates. The secondary outcome was that there is a correlation between flow rate and fluid viscosity.
Five infusion fluids commonly used in the prehospital environment were utilized: 0.9% normal saline 1,000 mL (JW Pharmaceutical, Seoul, Korea), Hartmann’s solution 1,000 mL (JW Pharmaceutical), Plasma Solution-A 1,000 mL (HK inno.N, Seoul, Korea), 6% hetastarch 500 mL (Fresenius Kabi Deutschland GmbH, Bad Homburg, Germany) and 5% albumin 250 mL (GC Pharma, Yongin, Korea). Viscosity (mPa·s) of each fluid was measured using a rotational viscometer (HAAKE MARS II; Thermo Fisher Scientific, Waltham, MA, USA) at a shear rate of 200/s at 20°C.
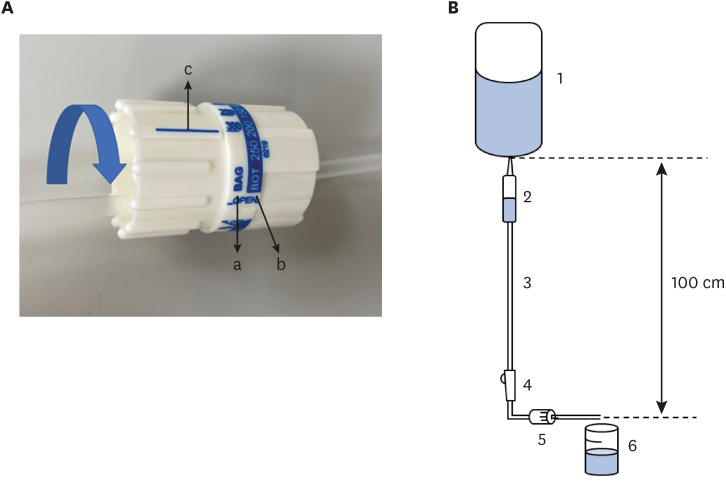
One type of IIFR (Infu-Green Plus Kit; Medi Line Active Korea, Ansan, Korea) was used. Two numeric scales were marked on the IIFR: one based on the infusion rate when the IIFR is connected to IV fluids in plastic bags, and the other for fluids in infusion bottles (
Fig. 1A). Therefore, the three types of crystalloids and 6% hetastarch were regulated based on the former, and 5% albumin was regulated based on the latter. The IIFR was connected to the IV fluid container suspended 100 cm above the distal end of tubing (
Fig. 1B) as recommended by the manufacturer’s instructions. The tip of tubing was placed horizontally and into a 100 mL beaker (Samduk-Lab, Guri, Korea). The IV tubing roller clamp was opened and the fluid was run for exactly 6 minutes. Trials were repeated five times in total for each type of fluid at three preset infusion rates (20, 100, or 250 mL/hr). The weight of the fluid was measured using an electronic scale (ABW04; Mettler Toledo, Columbus, OH, USA). The volume of the fluid was calculated indirectly by using the relative density (kg/L) of the fluids which had been previously measured by recording the weight of 50 mL of each type of fluid at 20°C, 1,023 hPa. Prior to the trials, Each IIFR was tested to be within ± 5% of the preset infusion rate when using 0.9% normal saline. The entire experiment was conducted by one researcher on the same day.
 | Fig. 1
The equipment for measuring flow rates. (A) Detailed picture of IIFR. Two types of numeric scales are marked on the IIFR dial. The scales marked as “BAG” indicated by arrow “a” are based on the flow rate of the normal saline contained in the IV fluid plastic bag. The scales marked as “BOT” indicated by arrow “b” are based on the flow rate of the normal saline contained in the infusion bottle. According to the manufacturer’s manual, if the blue arrow is rotated to match the scale with “c”, the fluid is injected at the flow rate (mL/hr) indicated by the number written on the scale. (B) Schematic presentation of the protocol. An IIFR was connected to an IV fluid bag, suspended 100 cm above the distal end of the IIFR. The distal tip of the IIFR was placed horizontally and into a beaker resting on a stable surface. The line of the IIFR was pre-flushed to eliminate air bubbles inside the line, and half of the drip chamber was filled. 1: IV fluid plastic bag or infusion bottle, 2: drip chamber, 3: tube, 4: roller clamp, 5: IV infusion flow regulator, 6: beaker.
IIFR = intravenous infusion flow regulator, IV = intravenous.

|
All statistical analyses were performed using IBM® SPSS® Statistics version 25 (IBM, Chicago, IL, USA). Nonparametric Kruskal-Wallis one-way analysis of variance was used to compare the infusion rate among the four types of IV fluids except 5% albumin, followed by post hoc testing using the Mann-Whitney U test adjusted by Bonferroni correction. Pearson's rank order correlation test was utilized to determine the correlation between viscosity and the infusion rate. This study had no ethical considerations.
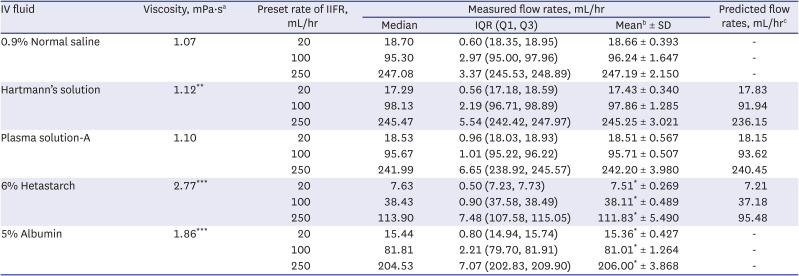
The viscosities and infusion rates of the IV solutions are shown in
Table 1. All three crystalloids showed similar viscosities (1.07–1.12 mPa·s). 6% hetastarch (2.77 mPa·s) was 2.59 times more viscous than normal saline, and 5% albumin (1.86 mPa·s) was 1.74 times more viscous.
Table 1
Viscosities and measured flow rates of IV fluids
|
IV fluid |
Viscosity, mPa·sa
|
Preset rate of IIFR, mL/hr |
Measured flow rates, mL/hr |
Predicted flow rates, mL/hrc
|
|
Median |
IQR (Q1, Q3) |
Meanb ± SD |
|
0.9% Normal saline |
1.07 |
20 |
18.70 |
0.60 (18.35, 18.95) |
18.66 ± 0.393 |
- |
|
100 |
95.30 |
2.97 (95.00, 97.96) |
96.24 ± 1.647 |
- |
|
250 |
247.08 |
3.37 (245.53, 248.89) |
247.19 ± 2.150 |
- |
|
Hartmann’s solution |
1.12**
|
20 |
17.29 |
0.56 (17.18, 18.59) |
17.43 ± 0.340 |
17.83 |
|
100 |
98.13 |
2.19 (96.71, 98.89) |
97.86 ± 1.285 |
91.94 |
|
250 |
245.47 |
5.54 (242.42, 247.97) |
245.25 ± 3.021 |
236.15 |
|
Plasma solution-A |
1.10 |
20 |
18.53 |
0.96 (18.03, 18.93) |
18.51 ± 0.567 |
18.15 |
|
100 |
95.67 |
1.01 (95.22, 96.22) |
95.71 ± 0.507 |
93.62 |
|
250 |
241.99 |
6.65 (238.92, 245.57) |
242.20 ± 3.980 |
240.45 |
|
6% Hetastarch |
2.77***
|
20 |
7.63 |
0.50 (7.23, 7.73) |
7.51* ± 0.269 |
7.21 |
|
100 |
38.43 |
0.90 (37.58, 38.49) |
38.11* ± 0.489 |
37.18 |
|
250 |
113.90 |
7.48 (107.58, 115.05) |
111.83* ± 5.490 |
95.48 |
|
5% Albumin |
1.86***
|
20 |
15.44 |
0.80 (14.94, 15.74) |
15.36* ± 0.427 |
- |
|
100 |
81.81 |
2.21 (79.70, 81.91) |
81.01* ± 1.264 |
- |
|
250 |
204.53 |
7.07 (202.83, 209.90) |
206.00* ± 3.868 |
- |

No significant difference in the measured infusion rates was found among crystalloids at the three preset infusion rates. However, the infusion rates were significantly decreased in 6% hetastarch (7.63, 38.43, and 113.90 mL/hr) compared with normal saline (P = 0.032, P = 0.032 and P = 0.032, respectively). 5% albumin also showed significantly lower infusion rates (15.44, 81.81, and 204.53 mL/hr) than normal saline (P = 0.032, P = 0.032 and P = 0.032, respectively). The flow rate of 6% hetastarch was approximately 60% slower than the preset flow rate, and 5% albumin was approximately 20% slower.
Based on the viscosity and actual flow of normal saline, measured flow rates of other crystalloids and 6% hetastarch in IV fluid plastic bag showed similar flow rate with predicted rate, corresponding to Poiseuille’s law (
Table 1). A negative correlation was found between the viscosities of the fluids and the measured flow rates. Pearson’s correlation coefficients were −0.993 (
P < 0.001), −0.998 (
P < 0.001), and −0.998 (
P < 0.001) when the IIFRs were set to 20, 100, and 250 mL/hr, respectively, indicating that flow rate decreases as viscosity increases.
In this study, crystalloids were delivered at a flow rate similar to the preset flow rate by the IIFRs, but colloids were delivered at a significantly lower rate than the preset rate. The results were consistent with previous studies in which the flow was reportedly inversely related to viscosity and that flow rates were lower with colloids than with crystalloids.
567 In addition to viscosity, pressure of the fluid can affect the flow rate. The flow rates were consistently slower than expected at lower heights and faster than expected at higher heights,
8 and therefore fluid containers must be hung at the height recommended by the IIFR’s manufacturer’s instructions.
The 6% hetastarch is a commonly used colloid in the operating room and ICU as a volume expander to treat hypovolemia in patients.
9 Albumin is also commonly administered in critically ill neonates under various clinical settings, such as sepsis, hypotension, delivery room resuscitation, and postoperative fluid management.
1011 Although the user’s guidelines of IIFR state that highly viscous solutions cannot be used, in actual clinical practice viscosity is commonly not taken into account, and IIFR is used for colloids without knowing the degree of discrepancy between the preset at actual flow rates. In the present study, less than half of the desired volume of 6% hetastarch was actually delivered when using an IIFR. The 5% albumin was also infused at 80% of the preset rate even being controlled by the IIFR scale for bottled fluid.
The present study had several limitations. The preset flow rates of IIFRs tested in this study were 20, 100, and 250 mL/h, which are commonly set flow rates for maintenance fluid therapy for infants, children, and adults, respectively. The effect of viscosity on flow rate under the setting of rapid resuscitation, such as when the IIFR is set to 1,000 mL/h, has yet to be studied, and further research is needed. Another limitation is that trials were conducted in vitro. The effects of viscosity on IIFRs have not been determined in an in vivo setting. So, further research is needed to confirm these effects in a clinical setting.
Based on the inaccuracy of the IIFRs observed in this study, it should be taken in to consideration that the performance of these devices is likely overestimated for fluid rate control in the patient care environment. The use of IIFR may not provide precise control of flow rates when infusing highly viscous solutions or fluids in infusion bottles. Scaling of the IIFR should be performed taking into account the viscosity of the fluids. If a certain and accurate amount of bottled fluid or colloid must be administered, the use of electronic IV infusion pumps might be a better option.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download