1. Committee Opinion No 701: choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017; 129:e155–9.
2. Johnson N, Barlow D, Lethaby A, Tavender E, Curr L, Garry R. Methods of hysterectomy: systematic review and meta-analysis of randomised controlled trials. BMJ. 2005; 330:1478.

3. O’Neill M, Moran PS, Teljeur C, O’Sullivan OE, O’Reilly BA, Hewitt M, et al. Robot-assisted hysterectomy compared to open and laparoscopic approaches: systematic review and meta-analysis. Arch Gynecol Obstet. 2013; 287:907–18.

4. Tulandi T, Krishnamurthy S, Mansour F, Suarthana E, Al-Malki G, Ballesteros LER, et al. A triple-blind randomized trial of preemptive use of gabapentin before laparoscopic hysterectomy for benign gynaecologic conditions. J Obstet Gynaecol Can. 2019; 41:1282–8.

5. Benton A, Harkins G, Stetter C, Kunselman A, Deimling T, Riley K. Administration of preoperative gabapentin to patients undergoing laparoscopy: a double-blinded, placebo-controlled randomized trial. J Gynecol Surg. 2020; 36:173–8.

6. As-Sanie S, Till SR, Mowers EL, Lim CS, Skinner BD, Fritsch L, et al. Opioid prescribing patterns, patient use, and postoperative pain after hysterectomy for benign indications. Obstet Gynecol. 2017; 130:1261–8.

7. Weston E, Raker C, Huang D, Parker A, Cohen M, Robison K, et al. Opioid use after minimally invasive hysterectomy in gynecologic oncology patients. Gynecol Oncol. 2019; 155:119–25.

8. Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, et al. Opioid complications and side effects. Pain Physician. 2008; 11(2 Suppl):S105–20.

9. Sills GJ. The mechanisms of action of gabapentin and pregabalin. Curr Opin Pharmacol. 2006; 6:108–13.

10. Bockbrader HN, Wesche D, Miller R, Chapel S, Janiczek N, Burger P. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet. 2010; 49:661–9.

11. Calandre EP, Rico-Villademoros F, Slim M. Alpha2delta ligands, gabapentin, pregabalin and mirogabalin: a review of their clinical pharmacology and therapeutic use. Expert Rev Neurother. 2016; 16:1263–77.

12. Dauri M, Faria S, Gatti A, Celidonio L, Carpenedo R, Sabato AF. Gabapentin and pregabalin for the acute postoperative pain management. A systematic-narrative review of the recent clinical evidences. Curr Drug Targets. 2009; 10:716–33.

13. Clarke H, Bonin RP, Orser BA, Englesakis M, Wijeysundera DN, Katz J. The prevention of chronic postsurgical pain using gabapentin and pregabalin: a combined systematic review and meta-analysis. Anesth Analg. 2012; 115:428–42.
14. Chang CC, Yen WT, Lin YT, Wang LK, Hung KC, Wu ZF, et al. Perioperative pregabalin for preventive analgesia in breast cancer surgery: a meta-analysis of randomized controlled trials. Clin J Pain. 2020; 36:968–77.
15. Hannon CP, Fillingham YA, Browne JA, Schemitsch EH, Mullen K, Casambre F, et al. The efficacy and safety of gabapentinoids in total joint arthroplasty: systematic review and direct meta-analysis. J Arthroplasty. 2020; 35:2730–8e6.

16. Yu Y, Liu N, Zeng Q, Duan J, Bao Q, Lei M, et al. The efficacy of pregabalin for the management of acute and chronic postoperative pain in thoracotomy: a meta-analysis with trial sequential analysis of randomized-controlled trials. J Pain Res. 2018; 12:159–70.

17. Wang SL, Wang H, Nie HY, Bu G, Shen XD, Wang H. The efficacy of pregabalin for acute pain control in herpetic neuralgia patients: a meta-analysis. Medicine (Baltimore). 2017; 96:e9167.
18. Ni J, Jiang J, Mao S, Sun RF. Pregabalin does not decrease acute pain or postoperative nausea and vomiting after hysterectomy: a meta-analysis. J Int Med Res. 2020; 48:300060520954720.

19. Asgari Z, Rouholamin S, Nataj M, Sepidarkish M, Hosseini R, Razavi M. Dose ranging effects of pregabalin on pain in patients undergoing laparoscopic hysterectomy: a randomized, double blinded, placebo controlled, clinical trial. J Clin Anesth. 2017; 38:13–7.

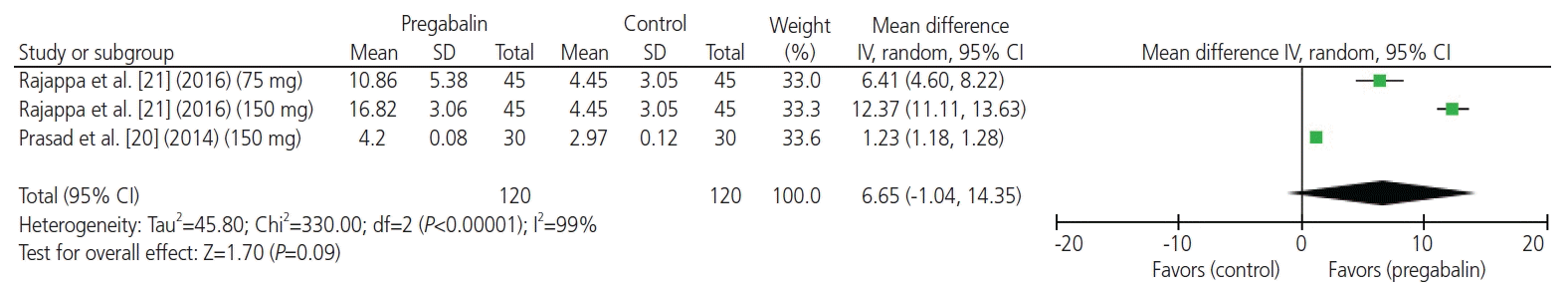
20. Prasad A, Bhattacharyya S, Biswas A, Saha M, Mondal S, Saha D. A comparative study of pre-operative oral clonidine and pregabalin on post-operative analgesia after spinal anesthesia. Anesth Essays Res. 2014; 8:41–7.

21. Rajappa GC, Vig S, Bevanaguddaiah Y, Anadaswamy TC. Efficacy of pregabalin as premedication for post-operative analgesia in vaginal hysterectomy. Anesth Pain Med. 2016; 6:e34591.

22. Sanguanwongthong K, Imruetaicharoenchok A, Phaloprakarn C, Vitayaburananont P. A randomized, double-blind, placebo-controlled trial of pre-emptive pregabalin for postoperative pain after laparoscopic hysterectomy in benign gynecologic diseases. J Med Assoc Thai. 2019; 102:39.
23. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6:e1000097.

24. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane handbook for systematic reviews of interventions version 5.1.0 [Internet]. London (UK): The Cochrane Collaboration;c2021. [cited 2011 Sep 4]. Available from:
www.handbook.cochrane.org
.
25. Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974; 2:656–9.

26. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928.

27. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–60.

28. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–34.

29. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014; 14:135.

30. Kutbi EH, Sohouli MH, Fatahi S, Lari A, Shidfar F, Aljhdali MM, et al. The beneficial effects of cinnamon among patients with metabolic diseases: a systematic review and dose-response meta-analysis of randomized-controlled trials. Crit Rev Food Sci Nutr. 2021. Mar. 19. [Epub].
https://doi.org/10.1080/10408398.2021.1896473
.

31. Abu-Zaid A, Altowairqi AK, Dissanayaka T, Oganesyan A, Bhagavathul AS, Alhabeeb H, et al. A systematic review and dose-response meta-analysis on the efficacy of dapagliflozin in patients with type 1 diabetes mellitus. Pharmacol Res. 2021; 165:105456.

32. Gottschalk A, Smith DS. New concepts in acute pain therapy: preemptive analgesia. Am Fam Physician. 2001; 63:1979–84.
33. Zhang D, You G, Yao X. Influence of pregabalin on postoperative pain after laparoscopic cholecystectomy: a meta-analysis of randomised controlled trials. J Minim Access Surg. 2019; 16:99–105.

34. Blanton E, Lamvu G, Patanwala I, Barron KI, Witzeman K, Tu FF, et al. Non-opioid pain management in benign minimally invasive hysterectomy: a systematic review. Am J Obstet Gynecol. 2017; 216:557–67.

35. Myles PS, Myles DB, Galagher W, Boyd D, Chew C, MacDonald N, et al. Measuring acute postoperative pain using the visual analog scale: the minimal clinically important difference and patient acceptable symptom state. Br J Anaesth. 2017; 118:424–9.

36. Hu J, Huang D, Li M, Wu C, Zhang J. Effects of a single dose of preoperative pregabalin and gabapentin for acute postoperative pain: a network meta-analysis of randomized controlled trials. J Pain Res. 2018; 11:2633–43.

37. White PF, Tufanogullari B, Taylor J, Klein K. The effect of pregabalin on preoperative anxiety and sedation levels: a dose-ranging study. Anesth Analg. 2009; 108:1140–5.

38. Li XD, Han C, Yu WL. Is gabapentin effective and safe in open hysterectomy? A PRISMA compliant meta-analysis of randomized controlled trials. J Clin Anesth. 2017; 41:76–83.

39. Noor N, Roy KK, Zangmo R, Das A, Rai R, Kumari A, et al. Role of para-cervical block in reducing immediate postoperative pain after total laparoscopic hysterectomy: a prospective randomized placebo-controlled trial. Obstet Gynecol Sci. 2021; 64:122–9.

40. Terrin N, Schmid CH, Lau J. In an empirical evaluation of the funnel plot, researchers could not visually identify publication bias. J Clin Epidemiol. 2005; 58:894–901.

41. Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006; 333:597–600.

42. Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000; 53:1119–29.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download