INTRODUCTION
MATERIALS AND METHODS
Data sources
Study design and subject selection
Fig. 1
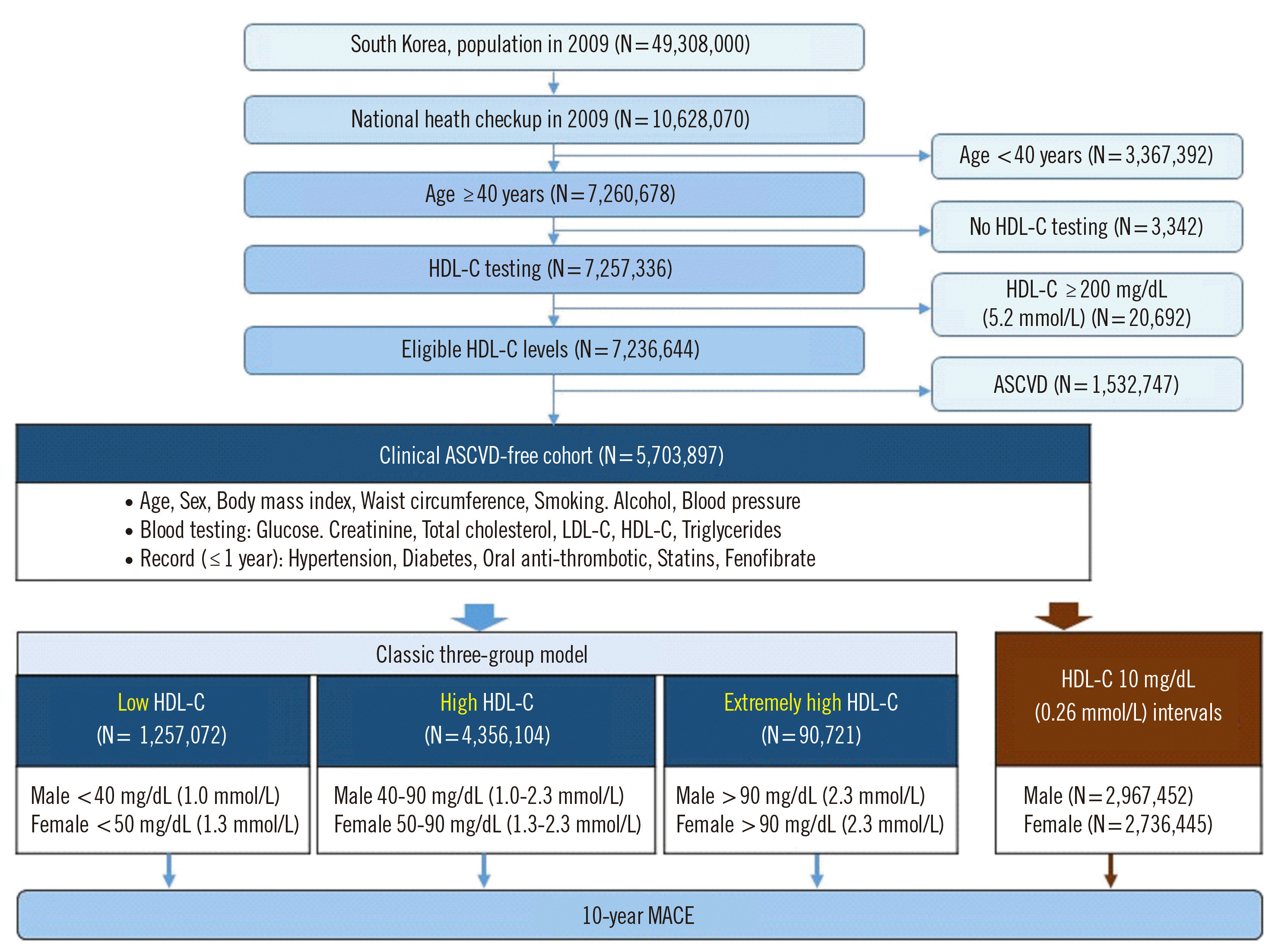
Definitions
Statistical analysis
RESULTS
Baseline characteristics
Table 1
| All subjects | Men | Women | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||||
| Total | Low HDL-C | High HDL-C | Extremely high HDL-C | Total | Low HDL-C | High HDL-C | Extremely high HDL-C | Total | Low HDL-C | High HDL-C | Extremely high HDL-C | |||
| Subjects, N (%) | 5,703,897 (100) | 1,257,072 (22.0) | 4,356,104 (76.4) | 90,721 (1.6) | 2,967,452 (100) | 444,690 (15.0) | 2,488,885 (83.9) | 33,877 (1.1) | 2,736,445 (100) | 812,382 (29.7) | 1,867,219 (68.2) | 56,844 (2.1) | ||
| Age (yr, mean ± SD) | 52.6 ± 9.8 | 53.9 ± 10.4 | 52.2 ± 9.6 | 52.2 ± 9.5 | 52.3 ± 9.7 | 53.5 ± 9.8 | 52.2 ± 9.6 | 53.7 ± 10.0 | 52.9 ± 10.0 | 54.7 ± 10.6 | 52.2 ± 9.6 | 51.4 ± 9.1 | ||
| 40-49, N (%) | 2,554,534 (44.8) | 500,666 (39.8) | 2,013,148 (46.2) | 40,720 (44.9) | 1,378,657 (46.5) | 202,147 (45.5) | 1,162,895 (46.7) | 13,615 (40.2) | 1,175,877 (43.0) | 298,519 (36.8) | 850,253 (45.5) | 27,105 (47.7) | ||
| 50-59, N (%) | 1,787,223 (31.3) | 391,571 (31.1) | 1,365,502 (31.3) | 30,150 (33.2) | 909,808 (30.7) | 137,705 (31.0) | 761,465 (30.6) | 10,638 (31.4) | 877,415 (32.1) | 253,866 (31.3) | 604,037 (32.4) | 19,512 (34.3) | ||
| 60-69, N (%) | 940,227 (16.5) | 240,100 (19.1) | 686,038 (15.7) | 14,089 (15.5) | 478,065 (16.1) | 72,033 (16.2) | 399,174 (16.0) | 6,858 (20.2) | 462,162 (16.9) | 168,067 (20.7) | 286,864 (15.4) | 7,231 (12.7) | ||
| 70-79, N (%) | 369,304 (6.5) | 107,356 (8.5) | 256,932 (5.9) | 5,016 (5.5) | 178,549 (6.0) | 28,923 (6.5) | 147,165 (5.9) | 2,461 (7.3) | 190,755 (7.0) | 78,433 (9.7) | 109,767 (5.9) | 2,555 (4.5) | ||
| ≥ 80, N (%) | 52,609 (0.9) | 17,379 (1.4) | 34,484 (0.8) | 746 (0.8) | 22,373 (0.8) | 3,882 (0.9) | 18,186 (0.7) | 305 (0.9) | 30,236 (1.1) | 13,497 (1.7) | 16,298 (0.9) | 441 (0.8) | ||
| BMI (kg/m2) | 23.8 ± 3.2 | 24.4 ± 3.0 | 23.7 ± 3.0 | 22.5 ± 8.6 | 24.1 ± 2.9 | 24.9 ± 2.8 | 23.9 ± 2.9 | 22.5 ± 2.9 | 23.6 ± 3.4 | 24.2 ± 3.1 | 23.3 ± 3.1 | 22.5 ± 10.6 | ||
| Obesity*, N (%) | 1,820,717 (31.9) | 494,192 (39.3) | 1,310,289 (30.1) | 16,236 (17.9) | 1,044,494 (35.2) | 206,081 (46.3) | 832,164 (33.4) | 6,249 (18.4) | 776,223 (28.4) | 288,111 (35.5) | 478,125 (25.6) | 9,987 (17.6) | ||
| Abd. obesity†, N (%) | 1,147,136 (20.1) | 332,098 (26.4) | 804,993 (18.5) | 10,045 (11.1) | 650,080 (21.9) | 135,461 (30.5) | 510,574 (20.5) | 4,045 (11.9) | 497,056 (18.2) | 196,637 (24.2) | 294,419 (15.8) | 6,000 (10.6) | ||
| Smoking, N (%) | ||||||||||||||
| Never | 3,493,447 (61.6) | 900,245 (72.1) | 2,530,014 (58.4) | 63,188 (70.0) | 894,553 (30.3) | 128,575 (29.1) | 755,887 (30.5) | 10,091 (29.9) | 2,598,894 (95.7) | 771,670 (95.7) | 1,774,127 (95.7) | 53,097 (94.0) | ||
| Past | 887,892 (15.7) | 127,371 (10.2) | 750,131 (17.4) | 10,390 (11.5) | 850,655 (28.8) | 117,001 (26.5) | 724,319 (29.3) | 9,335 (27.7) | 38,137 (1.4) | 10,370 (1.3) | 26,712 (1.4) | 1,055 (1.9) | ||
| Current | 1,285,914 (22.7) | 220,844 (17.7) | 1,048,396 (24.2) | 16,674 (18.5) | 1,206,288 (40.9) | 196,600 (44.5) | 995,359 (40.2) | 14,329 (42.5) | 79,626 (2.9) | 24,244 (3.0) | 53,037 (2.9) | 2,345 (4.2) | ||
| Alcohol, N (%) | ||||||||||||||
| None | 3,096,108 (55.0) | 864,014 (69.8) | 2,188,755 (50.9) | 43,339 (48.2) | 978,688 (33.3) | 189,543 (43.2) | 782,141 (31.7) | 7,004 (20.8) | 2,117,420 (78.6) | 674,471 (84.5) | 1,406,614 (76.5) | 36,335 (64.6) | ||
| One time/week | 1,037,853 (18.4) | 192,426 (15.6) | 830,175 (19.3) | 15,252 (17.0) | 675,267 (23.0) | 107,529 (24.5) | 562,385 (22.8) | 5,353 (15.9) | 362,586 (13.5) | 84,897 (10.6) | 267,790 (14.6) | 9,899 (17.6) | ||
| Two times/week | 667,238 (11.8) | 90,599 (7.3) | 565,254 (13.1) | 11,385 (12.7) | 547,510 (18.6) | 68,103 (15.5) | 473,047 (19.2) | 6,360 (18.9) | 119,728 (4.4) | 22,496 (2.8) | 92,207 (5.0) | 5,025 (8.9) | ||
| ≥ three times/week | 831,110 (14.8) | 90,576 (7.3) | 720,535 (15.7) | 19,999 (22.2) | 735,947 (25.1) | 73,830 (16.8) | 647,127 (26.3) | 14,990 (44.5) | 95,163 (3.5) | 16,746 (2.1) | 73,408 (4.0) | 5,009 (8.9) | ||
| SBP (mm Hg) | 123.5 ± 15.4 | 123.7 ± 15.5 | 123.4 ± 15.3 | 123.5 ± 16.2 | 125.6 ± 14.7 | 125.4 ± 14.6 | 125.6 ± 14.8 | 128.1 ± 16.2 | 121.2 ± 15.7 | 122.7 ± 15.9 | 120.5 ± 15.5 | 120.7 ± 15.5 | ||
| DBP (mm Hg) | 77.0 ± 10.2 | 76.8 ± 10.2 | 77.0 ± 10.3 | 77.0 ± 10.7 | 78.7 ± 10.0 | 78.6 ± 9.9 | 78.8 ± 10.0 | 80.1 ± 10.6 | 75.1 ± 10.2 | 75.8 ± 10.2 | 74.7 ± 10.1 | 75.2 ± 10.3 | ||
| Hypertension, N (%) | 1,503,715 (26.4) | 379,159 (30.2) | 1,102,303 (25.3) | 22,253 (24.5) | 831,042 (28.0) | 134,508 (30.2) | 685,872 (27.6) | 10,662 (31.5) | 672,673 (24.6) | 244,651 (30.1) | 416,431 (22.3) | 11,591 (20.4) | ||
| DM, N (%) | 620,744 (10.9) | 169,273 (13.5) | 443,664 (10.2) | 7,807 (8.6) | 384,756 (13.0) | 73,416 (16.5) | 306,958 (12.3) | 4,382 (12.9) | 235,988 (8.6) | 95,857 (11.8) | 136,706 (7.3) | 3,425 (6.0) | ||
| Blood test at baseline, mg/dL (mmol/L) | ||||||||||||||
| Glucose | 94 [87-104] | 95 [87-105] | 94 [87-104] | 94 [87-103] | 96 [88-107] | 97 [88-109] | 96 [88-107] | 97 [88-109] | 93 [86-101] | 93 [86-102] | 92 [85-100] | 92 [85-100] | ||
| 5.2 [4.8-5.8] | 5.3 [4.8-5.8] | 5.2 [4.8-5.8] | 5.2 [4.8-5.7] | 5.3 [4.9-5.9] | 5.4 [4.9-6.1] | 5.3 [4.9-5.9] | 5.4 [4.9-6.1] | 5.2 [4.8-5.6] | 5.2 [4.8-5.7] | 5.1 [4.7-5.6] | 5.1 [4.7-5.6] | |||
| sCr | 0.9 [0.8-1.0] | 0.8 [0.7-1.0] | 0.9 [0.8-1.0] | 0.9 [0.7-1.0] | 1.0 [0.9-1.1] | 1.0 [0.9-1.1] | 1.0 [0.9-1.1] | 1.0 [0.9-1.1] | 0.8 [0.7-0.9] | 0.8 [0.7-0.9] | 0.8 [0.7-0.9] | 0.8 [0.7-0.9] | ||
| 79.6 [70.7-88.4] | 70.7 [61.9-88.4] | 79.6 [70.7-88.4] | 79.6 [61.9-88.4] | 88.4 [79.6-97.3] | 88.4 [79.6-97.3] | 88.4 [79.6-97.3] | 88.4 [79.6-97.3] | 70.7 [61.9-79.6] | 70.7 [61.9-79.6] | 70.7 [61.9-79.6] | 70.7 [61.9-79.6] | |||
| TC | 197 [174-222] | 189 [166-214] | 199 [176-223] | 217 [194-242] | 196 [173-220] | 186 [163-210] | 197 [175-222] | 213 [190-238] | 198 [175-224] | 191 [167-217] | 200 [178-226] | 219 [196-244] | ||
| 5.09 [4.5-5.74] | 4.89 [4.29-5.53] | 5.15 [4.55-5.77] | 5.61 [5.01-6.26] | 5.07 [4.47-5.69] | 4.81 [4.22-5.43] | 5.09 [4.53-5.74] | 5.51 [4.91-6.16] | 5.12 [4.53-5.79] | 4.94 [4.32-5.61] | 5.17 [4.60-5.84] | 5.66 [5.07-6.31] | |||
| LDL-C | 116 [94-138] | 116 [94-139] | 116 [95-138] | 99 [75-123] | 114 [92-136] | 110 [88-133] | 115 [93-137] | 99 [75-123] | 118 [97-141] | 119 [97-142] | 118 [97-141] | 103 [80-127] | ||
| 3.0 [2.43-3.57] | 3.0 [2.43-3.59] | 3.0 [2.46-3.57] | 2.56 [1.94-3.18] | 2.95 [2.38-3.52] | 2.84 [2.28-3.44] | 2.97 [2.41-3.54] | 2.56 [1.94-3.18] | 3.05 [2.51-3.65] | 3.08 [2.51-3.67] | 3.05 [2.51-3.65] | 2.66 [2.07-3.28] | |||
| HDL-C | 53 [45-63] | 39 [36-45] | 57 [50-65] | 97 [93-104] | 50 [43-60] | 36 [33-38] | 52 [46-61] | 98 [94-106] | 56 [48-66] | 44 [40-47] | 61 [55-69] | 97 [93-104] | ||
| 1.37 [1.16-1.63] | 1.01 [0.93-1.16] | 1.47 [1.29-1.68] | 2.51 [2.41-2.69] | 1.29 [1.11-1.55] | 0.93 [0.85-0.98] | 1.34 [1.19-1.58] | 2.53 [2.43-2.74] | 1.45 [1.24-1.71] | 1.14 [1.03-1.22] | 1.58 [1.42-1.78] | 2.51 [2.41-2.69] | |||
| Triglycerides | 113 [78-167] | 142 [98-209] | 107 [75-155] | 77 [57-109] | 129 [89-191] | 179 [124-262] | 123 [86-179] | 87 [62-126] | 98 [70-141] | 126 [88-182] | 90 [66-125] | 73 [55-100] | ||
| 1.28 [0.88-1.88] | 1.6 [1.11-2.36] | 1.21 [0.85-1.75] | 0.87 [0.64-1.23] | 1.46 [1.0-2.16] | 2.02 [1.4-2.96] | 1.39 [0.97-2.02] | 0.98 [0.7-1.42] | 1.11 [0.79-1.59] | 1.42 [0.99-2.05] | 1.02 [0.75-1.41] | 0.82 [0.62-1.13] | |||
| Medications use at baseline, N (%) | ||||||||||||||
| Oral anti-thrombotic | 336,867 (5.9) | 93,526 (7.4) | 239,320 (5.5) | 4,021 (4.4) | 178,847 (6.0) | 32,479 (7.3) | 144,665 (5.8) | 1,703 (5.0) | 158,020 (5.8) | 61,047 (7.5) | 94,655 (5.1) | 2,318 (4.1) | ||
| Statins | 326,127 (5.7) | 81,053 (6.4) | 239,562 (5.5) | 5,512 (6.1) | 145,254 (4.9) | 22,194 (5.0) | 121,340 (4.9) | 1,720 (5.1) | 180,873 (6.6) | 58,859 (7.3) | 118,222 (6.3) | 3,792 (6.7) | ||
| Fenofibrate | 10,129 (0.18) | 2,962 (0.24) | 7,102 (0.16) | 65 (0.07) | 6,414 (0.2) | 1,357 (0.3) | 5,026 (0.2) | 31 (0.1) | 3,715 (0.14) | 1,605 (0.20) | 2,076 (0.11) | 34 (0.06) | ||
All baseline characteristics have P<0.0001 based on ANOVA or chi-square tests on the three groups (low, high, and extremely high HDL-C). In a post-hoc analysis, in all subjects, significant differences were found between all three classic subgroups, except for three variables (age, SBP, and DBP) between the high and extremely high groups. In a post-hoc analysis by sex, significant differences were found between all subgroups, except for use of alcohol twice per week in men (high vs. extremely high), use of statins in men (low vs. extremely high), never smoking in women (low vs. high), and sCr in women (high vs. extremely high).
Missing data are shown in Supplemental Data Table S1.
Abbreviations: SD, standard deviation; MACE, major adverse cardiovascular events; HDL-C, high-density lipoprotein cholesterol; n, number; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; DM, diabetes mellitus; sCr, serum creatinine; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol.
Classic three-group comparisons of 10-year MACE and all-cause mortality
Table 2
| Total (N=5,703,897) | Low HDL-C (N=1,257,072) | High HDL-C (N=4,356,104) | Extremely high HDL-C (N=90,721) | P | |
|---|---|---|---|---|---|
| 10-yr MACE | |||||
| All cohorts, N (%) | 1,341,669 (23.5) | 333,011 (26.5) | 988,579 (22.7) | 20,079 (22.1) | < 0.0001* |
| By sex, N (% of each sex) | |||||
| Men | 746,182 (25.2) | 124,908 (28.1) | 611,767 (24.6) | 9,507 (28.1) | < 0.0001† |
| Women | 595,487 (21.8) | 208,103 (25.6) | 376,812 (20.2) | 10,572 (18.6) | < 0.0001* |
| By age, N (% of each age subgroup) | |||||
| 40-49 yr | 327,168 (12.8) | 69,690 (13.9) | 252,406 (12.5) | 5,072 (12.5) | < 0.0001‡ |
| 50-59 yr | 413,224 (23.1) | 97,000 (24.8) | 309,759 (22.7) | 6,465 (21.4) | < 0.0001* |
| 60-69 yr | 355,175 (37.8) | 93,092 (38.8) | 256,890 (37.5) | 5,193 (36.9) | < 0.0001‡ |
| 70-79 yr | 205,191 (55.6) | 59,734 (55.6) | 142,673 (55.5) | 2,784 (55.5) | 0.82 |
| ≥ 80 yr | 40,911 (77.8) | 13,495 (77.7) | 26,851 (77.9) | 565 (75.7) | 0.35 |
| All-cause mortality | |||||
| All cohorts, N (%) | 304,426 (5.3) | 74,034 (5.9) | 224,967 (5.2) | 5,425 (6.0) | < 0.0001† |
| By sex, N (% of each sex) | |||||
| Men | 207,300 (7.0) | 35,411 (8.0) | 168,166 (6.8) | 3,723 (11.0) | < 0.0001* |
| Women | 97,126 (3.6) | 38,623 (4.8) | 56,801 (3.0) | 1,702 (3.0) | < 0.0001‡ |
| By age, N (% of each age subgroup) | |||||
| 40-49 yr | 37,338 (1.5) | 7,077 (1.4) | 29,444 (1.5) | 817 (2.0) | < 0.0001* |
| 50-59 yr | 58,971 (3.3) | 12,342 (3.2) | 45,400 (3.3) | 1,229 (4.1) | < 0.0001* |
| 60-69 yr | 83,191 (8.9) | 19,138 (8.0) | 62,515 (9.1) | 1,538 (10.9) | < 0.0001* |
| 70-79 yr | 92,910 (25.2) | 24,886 (23.2) | 66,632 (25.9) | 1,392 (27.8) | < 0.0001* |
| ≥ 80 yr | 32,016 (60.9) | 10,591 (60.9) | 20,976 (60.8) | 449 (60.2) | 0.90 |
Distribution of 10-year MACE stratified by HDL-C levels
Fig. 2
Exploration of sex-specific reference points of HDL-C levels in 10 mg/dL (0.26 mmol/L) intervals
Hazard ratio (HR) of 10-year MACE by reference ranges in each sex
Table 3
| Reference range for HDL-C, mg/dL (mmol/L) | Group variable | Unadjusted HR (95% CI) | P | Adjusted HR* (95% CI) | P* | |
|---|---|---|---|---|---|---|
| Men | ||||||
| Classic | 40-90 (1.03-2.33) | Low | 1.17 (1.16-1.17) | < 0.0001 | 1.10 (1.09-1.10) | < 0.0001 |
| Extremely high | 1.17 (1.14-1.19) | < 0.0001 | 1.09 (1.07-1.11) | < 0.0001 | ||
| Model I | 40-70 (1.03-1.81) | Low | 1.17 (1.16-1.18) | < 0.0001 | 1.10 (1.09-1.10) | < 0.0001 |
| Extremely high | 1.03 (1.03-1.04) | < 0.0001 | 1.02 (1.01-1.02) | 0.0003 | ||
| Model II | 40-90 (1.03-2.33) | Low | 1.17 (1.16-1.17) | < 0.0001 | 1.10 (1.09-1.10) | < 0.0001 |
| Extremely high | 1.17 (1.14-1.19) | < 0.0001 | 1.09 (1.07-1.11) | < 0.0001 | ||
| Women | ||||||
| Classic | 50-90 (1.29-2.33) | Low | 1.31 (1.30-1.31) | < 0.0001 | 1.06 (1.05-1.06) | < 0.0001 |
| Extremely high | 0.92 (0.90-0.94) | < 0.0001 | 0.99 (0.97-1.01) | 0.22 | ||
| Model I | 50-110 (1.29-2.84) | Low | 1.31 (1.30-1.32) | < 0.0001 | 1.06 (1.05-1.07) | < 0.0001 |
| Extremely high | 1.05 (1.00-1.11) | 0.047 | 1.05 (1.00-1.10) | 0.06 | ||
| Model II | 50-130 (1.29-3.36) | Low | 1.31 (1.30-1.32) | < 0.0001 | 1.06 (1.05-1.07) | < 0.0001 |
| Extremely high | 1.30 (1.18-1.42) | < 0.0001 | 1.14 (1.04-1.25) | 0.01 | ||
*Adjusted for age, BMI, LDL-C, triglycerides, hypertension, DM, smoking, and alcohol consumption; for variables, refer to Table 1.
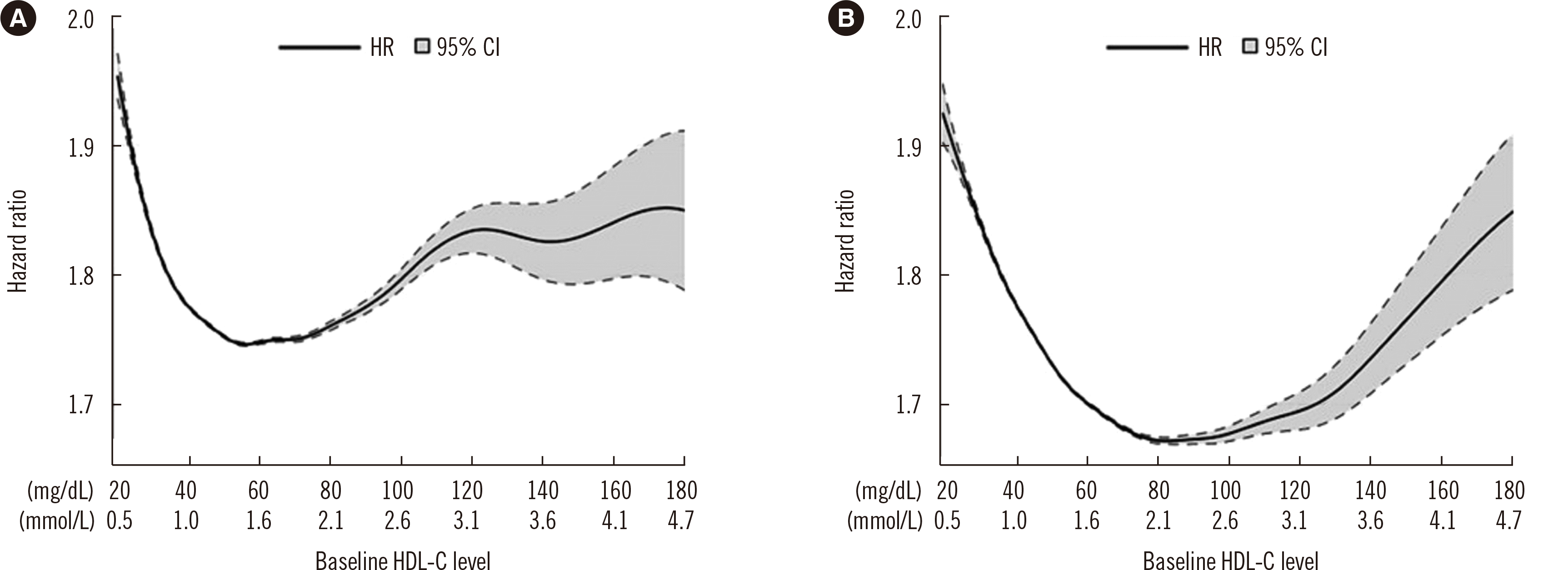
Cubic spline Cox proportional hazard regression model
Fig. 3




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download