INTRODUCTION
The number of patients with diabetes mellitus has increased substantially during the past few decades and is projected to continue to increase in the future [
1,
2]. Accurate information about insulin secretion, insulin resistance, and residual beta-cell function is needed for the therapy and classification of diabetes. Insulin and C-peptide measurements are the most frequently ordered laboratory tests to assess residual beta-cell function and insulin resistance for patients with diabetes [
3,
4]. Therefore, it is pivotal to harmonize insulin and C-peptide measurements among clinical laboratories. Such harmonization will facilitate the integration of information from numerous studies as well as the establishment of practical clinical guidelines to be used in most clinical settings.
Despite the increasing relevance of insulin and C-peptide measurements, studies have reported discordance among results obtained from different laboratories and different measurement systems; thus, substantial effort has been made to improve their harmonization status [
5-
8]. In 1996, the American Diabetes Association found that insulin measurements from different laboratories were widely discordant [
9]. In 2004, with the help of the National Institute of Diabetes and Digestive and Kidney Diseases of the United States, the European Association for the Study of Diabetes, the Center for Disease Control of the United States, and the International Federation of Clinical Chemistry and Laboratory Medicine, an American Diabetes Association workgroup was established to promote the comparability between insulin measurement results. In 2007, the working group reported improvement in the harmonization status of insulin measurements compared with that reported earlier [
9,
10]. However, this report also indicated that insulin measurements were still far from thorough standardization. A series of studies conducted by Manley,
et al. [
8] and Van Houcke,
et al. [
11] demonstrated that the comparability among laboratories could be improved using individual or pooled serum samples whose target values were determined by isotope dilution mass spectrometry (IDMS) to calibrate routine methods, which could help improve the traceability of manufacturers’ calibration systems to IDMS methods.
In 2002, the C-Peptide Standardization Committee sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases in the United States was established to promote the standardization of C-peptide measurements. The committee conducted an international comparison of C-peptide measurements in 2007, demonstrating that the results from different laboratories were not comparable [
12]. During the past few decades, considerable effort has been made to achieve harmonization between laboratories, which were comprehensively summarized by Little,
et al. in 2017 [
6].
Since the most recent studies investigating the harmonization status of insulin and C-peptide measurements were conducted in 2007, there is an urgent need to determine whether there has been improvement [
8,
12]. To assess the current harmonization status of serum insulin and C-peptide measurement of clinical laboratories in China, we compared the results of five pooled serum samples measured in 94 laboratories. The inter- and intra-laboratory %CVs as well as inter- and intra-measurement system %CVs were calculated and compared. To verify whether the poor comparability between laboratories remains linked to the calibrators of measurement systems, as reported previously [
6,
13,
14], low- and high-level serum samples were used to recalibrate results from different measurement systems, and the results before and after recalibration were compared.
MATERIALS AND METHODS
Samples
The set of five pooled serum samples were prepared from a large number of leftover samples collected from Beijing Hospital, Beijing, China, between August 2020 and January 2021. All samples were from patients undergoing insulin and C-peptide detections at Beijing Hospital clinical laboratory where the Siemens ADVIA Centaur XPT (Siemens Healthineers, Berlin, Germany) system was used. All samples were tested to ensure they did not react with anti-HIV antibodies, anti-hepatitis C virus antibodies, and hepatitis B virus surface antigen. Patient serum samples with hemolysis, icterus, or lipemia were considered deviant and not included for analysis. The Ethics Committee of Beijing Hospital approved this study and exempted the need for obtaining informed consent (approval number 2018BJYYEC-019-01). Collected patient serum samples were first divided into five groups (202111: insulin <0.06 nmol/L and C-peptide <0.25 nmol/L; 202112: insulin 0.10–0.30 nmol/L and C-peptide 0.50–0.70 nmol/L; 202113: insulin 0.30–0.40 nmol/L and C-peptide 1.50–1.90 nmol/L; 202114: insulin 0.40–0.50 nmol/L and C-peptide 2.00–2.30 nmol/L for C-peptide; and 202115: insulin >0.50 nmol/L and C-peptide >3.00 nmol/L) according to the primary results from Beijing Hospital. Samples in the same group were thawed, pooled overnight at 4°C on a magnetic stirring apparatus, and filtered using a vacuum pump (first with a 0.45-μm filter membrane and then with a 0.22-μm filter membrane). Each of the five pooled serum samples was split into aliquots of 0.5 mL in vials and stored at −80°C until shipment on dry ice to the participating laboratories. To ensure the homogeneity of aliquots of each pooled serum sample, complete mixing of the five pooled serum samples was ensured during the process of aliquoting using a magnetic stirring apparatus.
Analytical methods
One hundred eighty laboratories across China, which used various measurement systems (Roche, Abbott, Siemens, Beckman, Mindray, and Snibe;
Supplemental Data Table S1), signed up for this national investigation program. Given the limited aliquots of pooled serum samples, there was a demand to select a portion of the participating laboratories for subsequent measurements. The laboratories were first grouped according to their measurement systems to ensure a constant proportion of different systems used before and after the selection; the locations of selected laboratories were also chosen to be distributed as evenly possible across China. Ninety-four laboratories were ultimately selected. A registration form was provided to each selected laboratory requesting relevant information on reagents, calibrators, and measurement principles; other information, including the traceability of the measurement systems and the recommended reference interval, were provided by the manufacturers of the relevant measurement systems (
Supplemental Data Table S1).
Measurement protocol
The five pooled serum samples with three replicates each (total of 15 aliquots) were distributed on dry ice to the laboratories and stored at −80°C until measurement. The laboratories were instructed to measure one aliquot for each concentration two times a day at three specified days. All laboratory measurements were based on the protocols recommended by Chinese National Center for Clinical Laboratories for frozen serum samples. In brief, on the day of measurement, samples were let to stand at room temperature for approximately 20 minutes and mixed upside down 10 times. After reaching room temperature, each sample was measured twice consecutively. After converting the respective units into a standard unit (nmol/L), each laboratory was requested to submit six values for each pooled serum sample before the given deadline for a total of 30 values for the five serum samples.
Statistical analysis
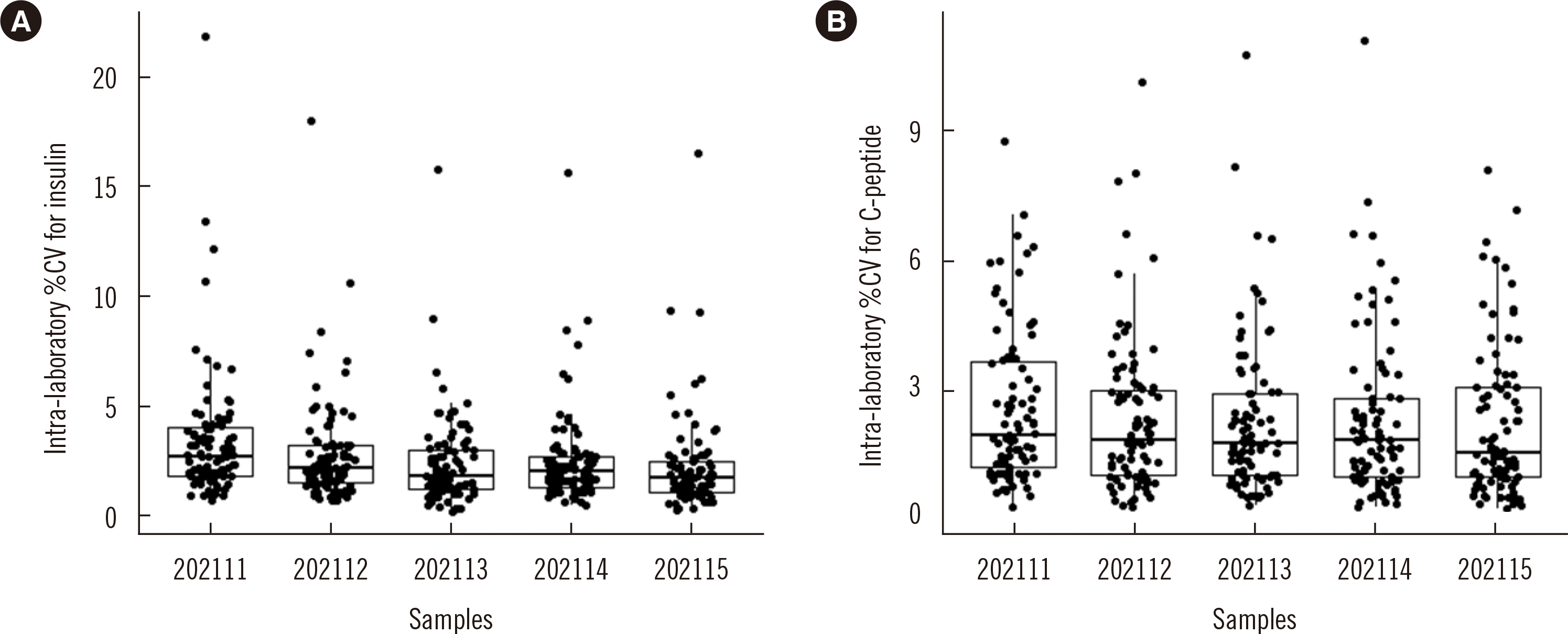
Data from laboratories that did not convert their units were removed prior to analysis. The six repeated values for each pooled serum sample from each laboratory were used to calculate the intra-laboratory imprecision (intra-laboratory %CV). The inter-laboratory imprecision (inter-laboratory %CV) was calculated using the mean of the six repeated values for each laboratory. Laboratories were divided into seven groups according to the measurement system used: Roche, Abbott, Siemens, Beckman, Mindray, Snibe, and others (including laboratories without information of measurement systems). The mean of laboratories in the same group was used to calculate the inter-measurement system imprecision (inter-measurement system %CV). The means of six repeated values of laboratories in the same group were used to calculate the intra-measurement system imprecision (intra-measurement system %CV).
The desirable analytical performance specifications (APS) for imprecision (10.6% for insulin, 8.3% for C-peptide) applied in this study were based on biological variation data and on the Westgard website (
https://www.westgard.com/biodatabase1.htm) [
15]. ANOVA was used to explore differences of inter-laboratory %CVs between concentrations; differences were considered to be statistically significant at
P<0.05.
To determine whether differences between measurement systems were correlated with the respective calibrators, two of the five pooled serum samples with the lowest and highest concentrations (202112 and 202115) were used to recalibrate the results of the other samples (202111, 202113, and 202114). The mean results of all measurement systems were set as the target values of the recalibration materials (202112 and 202115). The means of all laboratories using the same measurement system were regarded as the representative value of that measurement system. The target values as well as the representative values of samples 202112 and 202115 were used to draw the new standard curves for the six measurement systems. The recalibrated representative values of the six measurement systems for the other samples (202111, 202113, and 202114) were then calculated by placing the original representative values onto the new standard curve. The recalibrated representative values were used to calculate the recalibrated inter-measurement system %CV, which was compared with that calculated before recalibration. All calculations were performed in Microsoft Excel 2016 (Microsoft Corporation, Redmond, Washington, USA), R language, and MedCalc statistical software 18.11.6-64-bit (Mariakerke, Belgium).
DISCUSSION
On the basis of repeated results of five pooled serum samples from 94 laboratories, most intra-laboratory %CVs met the APS requirements for both analytes, demonstrating the generally good intra-laboratory precision for insulin and C-peptide measurements in clinical laboratories. However, the insulin and C-peptide measurements from different laboratories were not comparable, with inter-laboratory %CVs ranging from 8.4% to 64.3% for insulin and from 13.6% to 21.8% for C-peptide. The intra- and inter-laboratory %CVs increased with decreasing insulin concentration.
Accurate measurement of insulin at low concentration is clinically important for assessment of remaining beta-cell secretory function in type 2 diabetes as well as for assessing the insulin resistance of obese and non-obese patients. The obvious discordance among laboratories at low concentrations may result in contradictory testing interpretations. Similar to insulin, samples with a low C-peptide concentration had much higher inter-laboratory %CVs than those of samples with higher concentrations. We hypothesized that cross-reactions caused by various interferents such as pro-insulin or the metabolites of C-peptide would impact the comparability among different laboratories and measurement systems. Disparate degrees of cross-reactions were often observed for various measurement systems due to their different specificities, resulting in discordance between results from laboratories using different measurement procedures for the same set of samples, especially for samples with low concentrations of C-peptide.
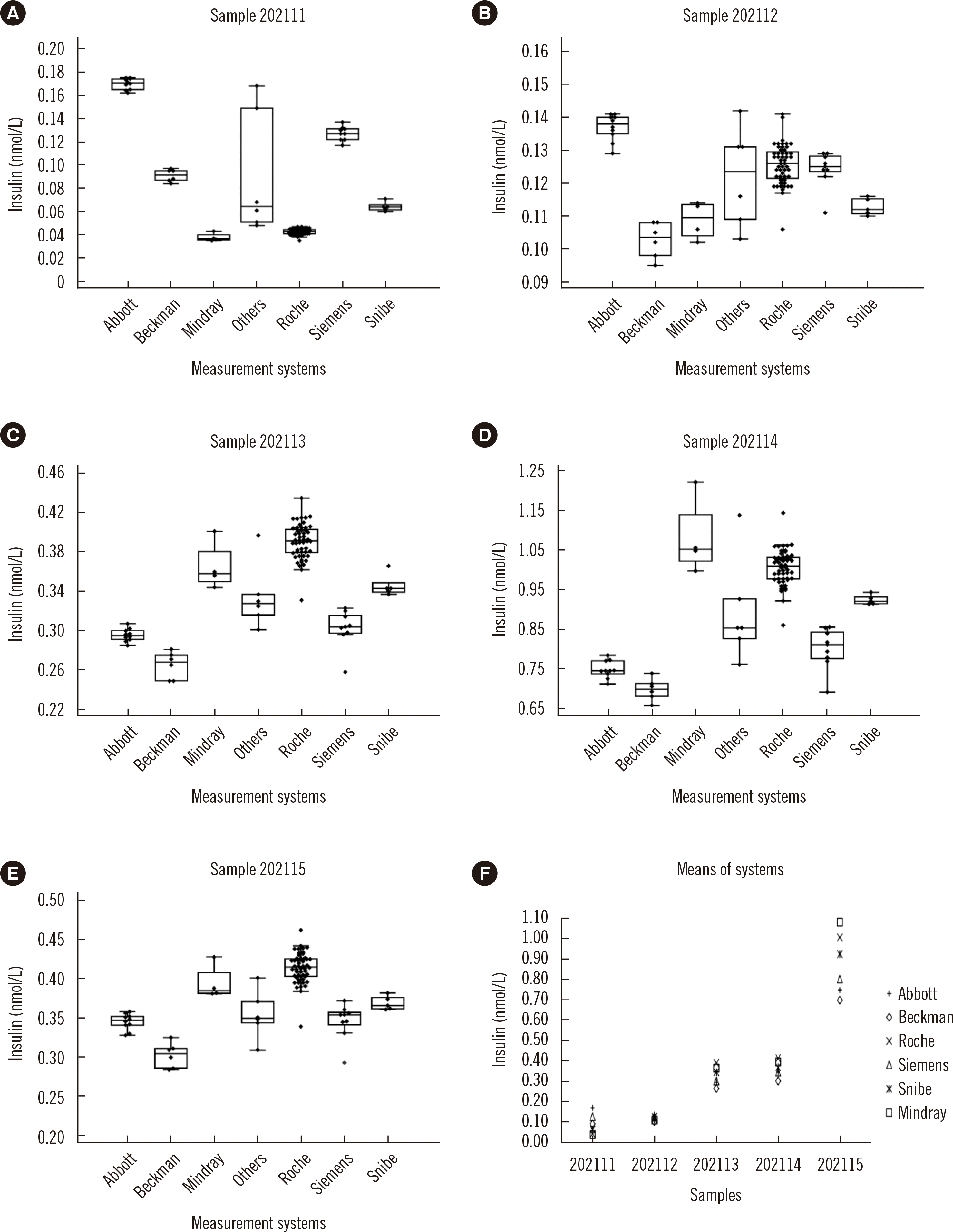
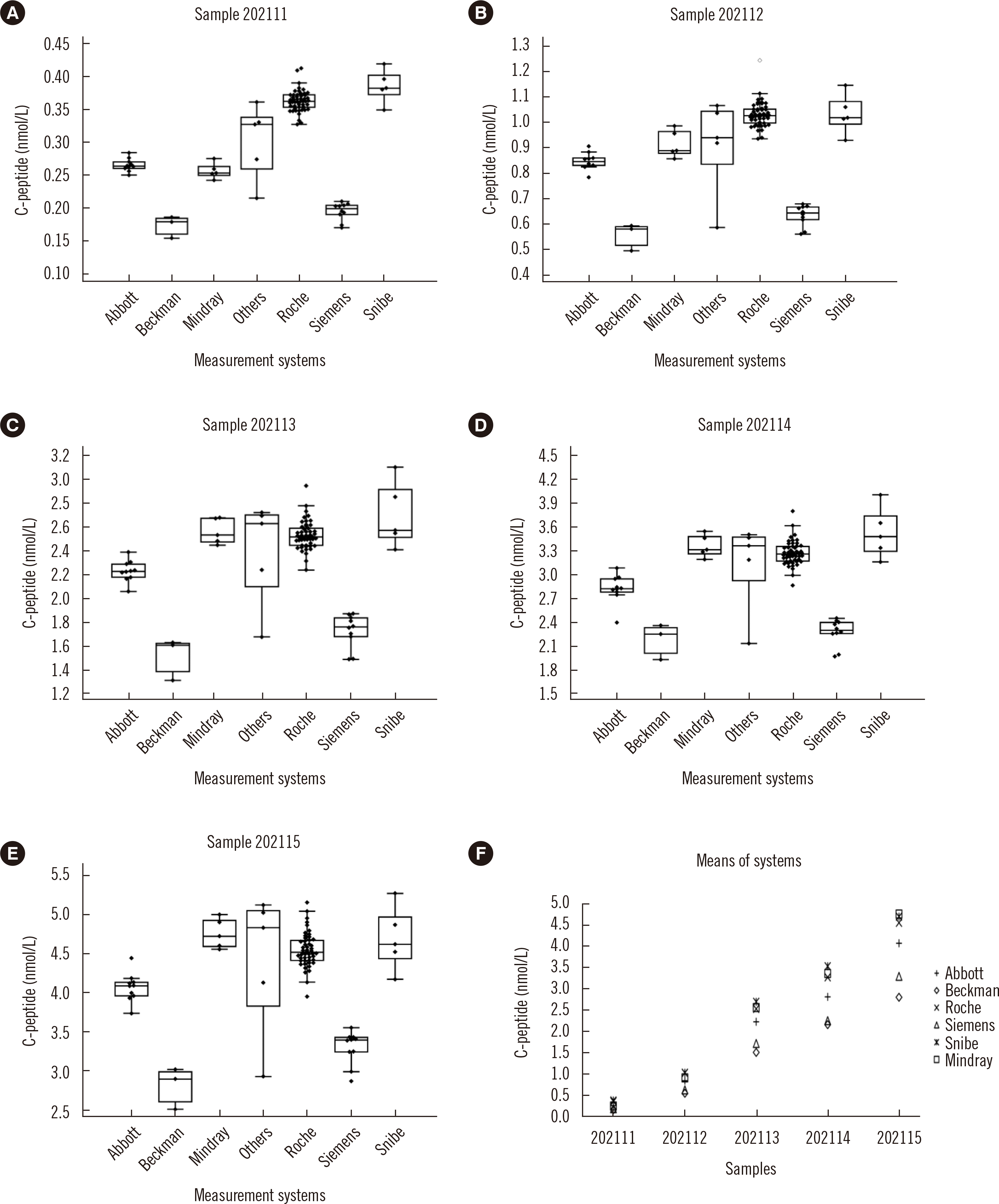
This study found good comparability among laboratories using the same measurement systems for measurements of insulin and C-peptide with respective mean intra-measurement systems %CV of 4.8% and 6.6%. However, the results of different measurement systems were not comparable, with inter-measurement system %CVs ranging from 10.7% to 58.3% for insulin and from 20.1% to 31.6% for C-peptide.
Although almost all of the manufacturers of the measurement systems included in this study claimed that their calibrators traced to the same WHO international reference reagents (WHO IRR 84/510 for C-peptide and 66/304 for insulin, which are both pure substances), except for Beckman whose calibrator for C-peptide traced to the WHO’s first international standard 13/146, the comparability among different measurement systems was not satisfactory. A likely explanation is that, as polypeptides, C-peptide and insulin may behave differently in pure solution than in a sample-matrix solution. The first international standard for human C-peptide (WHO ISR 13/146) and its international reference reagent (WHO IRR84/510) were reported to be incommutable for some measurement systems [
12,
16]. Comparability of C-peptide and insulin measurements between laboratories and measurement systems was effectively improved using one low-level and one high-level pooled sample to recalibrate clinical measurement systems, as reported previously [
13,
14].
Much work is still needed to achieve standardization of C-peptide and insulin measurements. For C-peptide, reference procedures and primary reference materials are listed in the Joint Committee for Traceability in Laboratory Medicine (JCTLM) dataset [
17-
19]. However, no commercially qualified secondary calibrators (usually sample matrix) are listed in the JCTLM dataset. Using serum samples assigned by a reference measurement procedure to calibrate clinical methods was corroborated as an effective approach to promote comparability among laboratories [
14]. The provision of patient serum samples to manufacturers according to a reference measurement procedure is an alternative acceptable mechanism to trace the results of each measurement system to the reference measurement procedure and the conversion to SI units [
6]. Recently, the C-peptide Standardization Committee organized the “C-Peptide Standardization Manufacturer Meeting” [
20], including C-peptide Standardization Committee members, manufacturer representatives, and other experts in clinical laboratory medicine. The C-peptide Standardization Committee recommended that manufacturers use pooled serum or single donor samples whose target values determined by the reference measurement procedure to recalibrate their measurement systems for C-peptide. The committee and manufacturers are actively working to overcome the many hurdles in recalibration of patient samples according to a reference measurement procedure. This initiative will greatly improve the standardization status of C-peptide, which is expected to be achieved in the near future.
For insulin, no reference measurement procedure and reference materials are listed in the JCTLM dataset. To improve the harmonization and standardization of insulin measurement, the unbroken traceability chain, including the reference laboratory, reference measurement procedure, reference measurement service, and qualified secondary calibrators, should be developed. Another recommended approach to calibrate clinical measurement procedures is to use patient-derived secondary reference materials such as pooled or single-donor serum or plasma with values determined by higher-order measurement procedures such as isotope diluent liquid-phase tandem mass spectrometry (ID-LC-MS/MS) [
14,
21].
External quality assessment (EQA) programs can provide valuable information about inter-laboratory imprecision, enabling the assessment and monitoring of the comparability among laboratories. However, the EQA materials for C-peptide and insulin in China are non-patient-derived lyophilized powders with unknown commutability, making it hard to compare and improve the between-method variations found in the EQA program. Since the commutability of patient-derived pooled serum samples is much better than that of non-patient-derived lyophilized powder, we used pooled serum samples in this study, as a more accurate method to recognize discordance among laboratories.
Despite the decrease in the inter-measurement system %CV of sample 202111 for insulin from 58.3% to 44.1% after recalibration, it still did not meet the APS specification of 10.6%, suggesting an influence other than traceability to explain the large inter-measurement system %CV for samples with a low concentration of insulin. This high variability might also be associated with instrumental discrepancy such as the poor specificity and sensitivity of the measurement systems for low-concentration measurements. Exogenous insulin and insulin analogs are commonly employed treatments for patients with diabetes. However, different immunoassays from different manufacturers usually use different anti-insulin antibodies, which may differentially combine with exogenous insulin and insulin analogs. Because all of the serum samples in this study were collected from patients who might be treated with various types of exogenous insulin and insulin analogs, sample 202111 might have contained a matrix similar to an insulin analog and/or exogenous insulin, which may have impacted the insulin measurement and contributed to the discordance among results from different measurement systems.
There are some limitations to our study. First, despite collection of a large number of measurement results from 94 laboratories, the small sample sizes limited our analysis; for example, only two of five pooled serum samples were used to recalibrate the remaining three samples, which may be insufficiently representative. Second, to reflect the true proportion of each manufacturer in Chinese hospitals, we attempted to maintain the proportions of different measurement systems in the 94 selected laboratories before and after the selection. However, some systems were only used in few laboratories (
Supplemental Data Table 1), and our results may not be sufficiently representative of this fact.
In conclusion, this study provides reliable data about laboratory performance as well as the standardization and harmonization status for serum insulin and C-peptide measurements. These data may provide support for laboratories to choose appropriate measurement systems to improve performance and can guide clinicians to better interpret the measurement results of serum insulin and C-peptide. We also demonstrated that commutable serum samples used as calibrators were effective in improving the comparability of serum C-peptide and insulin measurements obtained from different laboratories and measurement systems, as reported previously [
6,
13,
14].




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download