Abstract
Going back to basics prior to mentioning the use of antipsychotics in patients with pain, the International Association for the Study of Pain (IASP) definition of pain can be summarized as an unpleasant experience, composed of sensory experience caused by actual tissue damage and/or emotional experience caused by potential tissue damage. Less used than antidepressants, antipsychotics have also been used for treating this unpleasant experience as adjuvant analgesics without sufficient evidence from research. Because recently developed atypical antipsychotics reduce the adverse reactions of extrapyramidal symptoms, such as acute dystonia, pseudo-parkinsonism, akathisia, and tardive dyskinesia caused by typical antipsychotics, they are expected to be used more frequently in various painful conditions, while increasing the risk of metabolic syndromes (weight gain, diabetes, and dyslipidemia). Various antipsychotics have different neurotransmitter receptor affinities for dopamine (D), 5-hydroxytryptamine (5-HT), adrenergic (α), histamine (H), and muscarinic (M) receptors. Atypical antipsychotics antagonize transient, weak D2 receptor bindings with strong binding to the 5-HT2A receptor, while typical antipsychotics block long-lasting, tight D2 receptor binding. On the contrary, antidepressants in the field of pain management also block the reuptake of similar receptors, mainly on the 5-HT and, next, on the norepinephrine, but rarely on the D receptors. Antipsychotics have been used for treating positive symptoms, such as delusion, hallucination, disorganized thought and behavior, perception disturbance, and inappropriate emotion, rather than the negative, cognitive, and affective symptoms of psychosis. Therefore, an antipsychotic may be prescribed in pain patients with positive symptoms of psychosis during or after controlling all sensory components.
Go to : 
The International Association for the Study of Pain (IASP) definition of pain can be summarized as an unpleasant experience, composed of sensory experience caused by actual tissue damage and/or emotional experience caused by potential tissue damage [1]. During and after treating all sensory components including nociceptive and neuropathic pain, patients may complain of mysterious or vague generalized pain which are supposed to originate from the component of emotional experience.
Pain physicians are accustomed to use antidepressants for treating not only negative neuropathic pain symptoms, such as hypoesthesia/anesthesia and weakness, but also depressive mood. Anxiolytics, which reduce anxiety, and produce sedation and sleep from the lower to higher dose, are also familiar to them. However, they hesitate to use antipsychotics due to their major adverse reactions (ADRs) such as extrapyramidal symptoms (EPSs), even though the mechanisms of action, mainly acting on serotonin, norepinephrine, and dopamine, are quite similar.
Psychiatric symptoms and disorders include delirium, schizophrenia, delusional disorder, mood disorder, dementia, substance abuse, metabolic disturbances, chronic medical conditions, or drug-induced psychosis [23].
Antipsychotics are medications which are primarily used t o manag e schizophrenia a nd b ipolar d isorder. Antipsychotics have been prescribed for treating the 4 major symptoms of schizophrenia: positive (delusion, hallucination, disorganized thought and behavior, perception disturbance, and inappropriate emotion), negative (flat affect, poverty of thought, amotivation, and social withdrawal), cognitive (distractibility, impaired working memory, and impaired executive function), and affective (mania and depression) symptoms. However, antipsychotics are more e ffective o n positive s ymptoms than n eg ative symptoms. Mood symptoms are better controlled by antidepressants, and no drug treatment has been proven effective in cognitive deficits [234].
Typical antipsychotics have been tried in many intractable and unresponsive pain states as analgesics or adjuvants, however, adverse reactions (ADRs), such as extrapyramidal symptoms (EPSs) and sedation, would follow [56]. Chlorpromazine and haloperidol are representative typical antipsychotics. Chlorpromazine is one of the phenothiazine derivatives, and was originally developed during the development of the lytic cocktail for the prevention of surgical shock. As it showed decreased motor activity and affective indifference, it was used as an antipsychotic [7]. Haloperidol was a byproduct of meperidine during the search for a more powerful analgesic. It was found to have the effect of reducing an emotional crisis to the state of sedation. It is also effective to decrease emesis after an operation or opioid prescription [7].
One of the problems in prescribing conventional typical antipsychotics is that they can produce many ADRs. Depending on their actions with dopaminergic, adrenergic, cholinergic, serotonergic, and histaminergic receptors, they can present various ADRs. The representative ADRs of typical antipsychotics are EPSs, such as acute dystonia, pseudo-parkinsonism, akathisia, and tardive dyskinesia. Other ADRs are anticholinergic effects, orthostatic hypotension, seizure, and prolactin elevation. The ADRs, including EPSs caused by typical antipsychotics, had spurred the development of atypical antipsychotics, starting with clozapine in 1958 [7]. Recently developed atypical antipsychotics decrease the above ADRs, but still they increase the potential risk of metabolic syndromes (weight gain, diabetes, and dyslipidemia).
This article provides the classification, action mechanisms, clinical application (favorable symptoms), their equivalent doses, and ADRs of antipsychotics and their treatment of antipsychotics.
Go to : 
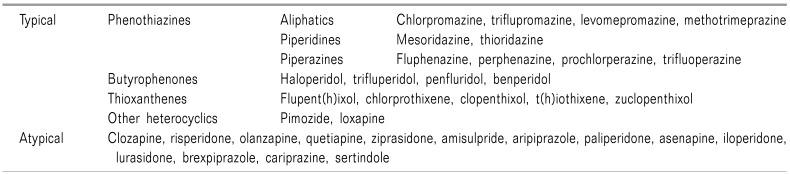
Antipsychotics can be classified as old, conventional, typical, or first generation antipsychotics (FGAs), and modern, novel, atypical or second generation antipsychotics (SGAs) (Table 1). They are classified according to their development and release, as well as their actions and ADRs.
Typical antipsychotics, FGAs, can be chemically classified as phenothiazine, butyrophenone, thioxanthene, and other heterocyclics.
Chlorpromazine from the phenothiazine class, was synthesized and used clinically in 1952, as the first classical antipsychotic [7].
Haloperidol, in the butyrophenone class, was synthesized during the development of analgesics from meperidine (1958).
Then, trifluoperazine (Eskazynil®, Stelazine®, 1959), thioridazine (Mellaril®, Melleril®, 1959 till withdrawal in 2005 due to cardiac arrhythmia), and fluphenazine (Prolixin®, Modecate®, Moditen®, 1959), a class of phenothiazines, came into use successively [78].
Flupent(h)ixol (Depixol®, Fluanxol®) from the thioxanthene class, was introduced in 1965 [9].
At least 17 atypical antipsychotics, SGAs, have been introduced a nd s ome drug s have a lready b een withdrawn because of ADRs. Clozapine (Clozaril®) is the prototypic atypical antipsychotic drug. It was synthesized in 1958 and tried clinically. But it turned out to be effective, with fewer neurologic ADRs for psychosis in 1966. It was launched in Europe, then introduced in the United States in 1989.
‘Atypical’ means these kinds of drugs don't produce EPSs that all the conventional antipsychotics possessed. Therefore, these new modern antipsychotics were described as ‘atypical’ [7].
That atypical drug development was followed by the introduction to the market of risperidone (Resperdal®, 1994), olanzapine (Zyprexa®, 1996), quetiapine (Seroquel®, 1998), ziprasidone (Geodon®, 2001), amisulpride (Solian®, 2002), aripiprazole (Abilify®, 2002), paliperidone (Invega®, 2006), asenapine (Saphris®, Sycrest®, 2009), iloperidone (Fanapt®, Fanapta®, 2009), lurasidone (Latuda®, 2010), sertindole (Serdolect®, Serlect®, 2013), brexpiprazole (Rexulti®, 2015), and cariprazine (Vraylar®, Reagila®, 2015) [8].
Antipsychotics have different receptor affinities for many neurotransmitters. It is many decades since chlorpromazine and haloperidol, the classic FGAs, were introduced and used clinically, and it is well established that their effects are via their action on dopamine receptors. By the increased level of monoamines, the classic FGAs were thought to act through increasing the turnover of monoamines and blocking monoamine receptors [10].
It is essential to understand the relevance of the classic concept of psychosis with 4 dopamine pathways: ⓐ mesolimbic pathways, related to positive symptoms (reward and pleasure), from the ventral tegmental area (VTA) located close to the midline on the floor of the midbrain, to the limbic system (including the amygdala and hippocampus), ⓑ mesocortical pathways, associated with negative, cognitive, and affective symptoms (motivation and emotions), from the VTA to the cortex, ⓒ nigrostriatal pathways, related to voluntary movement resulting in EPSs, from the substantia nigra pars compacta (the basal ganglia structure located in the midbrain, that plays an important role in reward and movement) to the dorsal striatum (the caudate nucleus and putamen, involved in the production of movement), ⓓ tuberoinfundibular pathways, related to hyperprolactinemia, from the infundibular nucleus (arcuate nucleus) in the tuberal region of the hypothalamus to the median eminence of the hypothalamus that regulates secretion of prolactin from the anterior pituitary gland [1112].
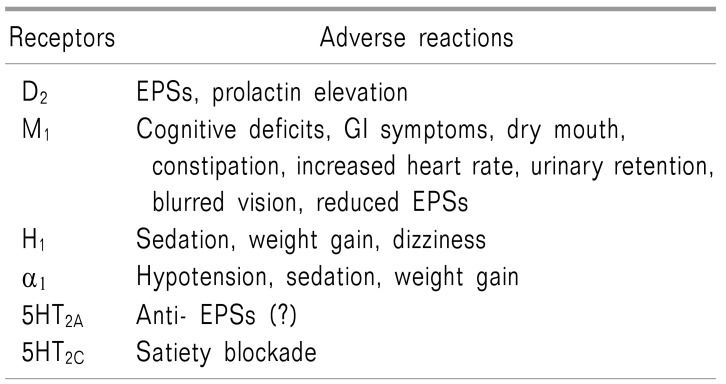
Making a connection with the 4 dopaminergic pathways relevant to the FGAs, the mechanisms of their action are antagonism to the D2 receptor in the brain, including ⓐ blockade of the mesolimbic pathway hyperactivation, related to the positive symptoms of psychosis, ⓑ hypofunction or dysfunction of the mesocortical pathway, related to negative, affective, and cognitive symptoms, ⓒ blockade of the nigrostriatal pathway, associated with increased risk of EPSs, and ⓓ blockade of the tuberoinfundibular pathway, engaged in increased prolactin levels in the pituitary gland. Therefore, the blockade of the mesolimbic and mesocortical pathways is deeply related to the therapeutic effects of the FGAs, however, blockade of the nigrostriatal and tuberoinfundibular pathways are used to explain the ADRs of FGAs (Table 2) [1112].
Matching the a ction mechanisms o f FGAs u p with EPSs, the representative ADRs, are explained by the blockade of the D2 receptors in the nigrostriatal pathway. It i s also very important to recog nize EPSs in an early phase for the patients taking FGAs. The drug-induced extrapyramidal symptoms scale (DIEPSS) is composed of 8 symptoms as well as the overall severity with their 5 level grading system [0 (normal), 1 (minimal), 2 (mild), 3 (moderate), and 4 (severe)]: ① gait, ② bradykinesia, ③ sialorrhea, ④ muscle rigidity, ⑤ tremors, ⑥ akathisia, ⑦ dystonia, ⑧ dyskinesia, and ⑨ overall severity [131415].
The representative EPSs are acute dystonia, pseudo-parkinsonism, akathisia, and tardive dyskinesia (Table 2). The pyramidal tract transmits motor s ignals through the anterior and lateral corticospinal or corticobulbar tract directly. However, the extrapyramidal system modulates or reg ulates motor s ig nals (involuntary movement with reflexes, locomotion, complex movements, and postural control) indirectly, without prominent innervating motor neurons (they are supposed to transmit through the rubrospinal, reticulospinal, olivospinal, or vestibulospinal tract related to the nigrostriatal pathway, basal ganglia of the subcortical and midbrain structures, cerebellum, and sensory area of the cerebral cortex) [16].
Dystonia is a movement disorder characterized by sustained or intermittent muscle contractions, causing abnormal posture, and/or repetitive movements [1718]. The common FGA-induced acute dystonia includes facial grimacing, involuntary upward eye movement, muscle spasms of the tongue, face, neck, and back, as well as laryngeal spasms. Acute dystonia is the earliest EPS, starting from a few hours to 5 days after administration. It usually reverses from a few hours to 1–2 days after discontinuation. It occurs in less than 10% of cases, but may develop in up to 40% in young male patients [16]. FGA-induced acute dystonia, although self-limiting, can be treated with antihistamines (such as diphenhydramine) or anticholinergics [1–6 mg of benz(a)tropine] initially, and benzodiazepines later, in cases of ineffectiveness.
Pseudo-parkinsonism, observed weeks to months after administration of FGAs, presents similar symptoms and signs to Parkinson's disease, as a neurodegenerative disease. However, differences between parkinsonism and pseudo-parkinsonism may lie in bradykinesia versus apraxic slowness, resting tremors versus essential tremors/myoclonus, lead pipe rigidity versus paratonic rigidity, postural instability versus frontal ataxia, and slow, shuffling gait with festination and retropulsion versus slow, shuffling apraxic gait, respectively [19]. Treatment includes anticholinergics or (100–400 mg/d of) amantadine, lowering the dose of antipsychotics, and changing to SGAs.
Akathisia refers to a subjective feeling of restlessness with anxiety and objective repetitive or restless motor activity days to weeks after administration. It is believed to develop due to a blockade of the mesocortical pathways and increased norepinephrine in the limbic region. Treatment includes beta-blockers (30–120 mg of propranolol), anticholinergics, benzodiazepines, lowering the dose of antipsychotics, and chang ing to SGAs [18].
Tardive dyskinesia refers to involuntary, repetitive hyperkinetic movements, including grimacing, sticking out the tongue, or smacking of the lips resulting from long-term use (–5 years: 31.8%, –10 years: 49.4%, –15 years: 56.7%, –20 years: 64.7%, 25– years: 68.4%) of FGAs or aripiprazole (SGA) because of supersensitivity in the nigrostriatal pathway [20].
The abnormal involuntary movement scale (AIMS) is the most popular method for evaluating drug-induced tardive dyskinesia. It is composed of 14 items from evaluating 5 different body areas, including ① orofacial movements (muscles of facial expression/lips and perioral area/jaw/tongue, 0 = none, 1 = minimal, 2 = mild, 3 = moderate, 4 = severe), ② extremity movements (upper extremities/lower extremities, from 0 to 4), ③ trunk movements (neck, shoulders, and hips, from 0 to 4), ④ global judgements (overall severity of abnormal movements/incapacitation due to abnormal movements/patient's awareness of abnormal movements, from 0 to 4), ⑤ dental status (current problems with teeth and/or dentures/worn dentures/edentia/do movements disappear with sleep?, 0 = no or 1 = yes) [21].
Valbenazine (12.5–100 mg/d) for 6 weeks is the first FDA-approved treatment for tardive dyskinesia. It causes a reversible reduction of dopamine release by selectively inhibiting presynaptic human vesicular monoamine transporter type 2 (VMAT2) [22].
Core features of delirium are disturbance of consciousness, change in cognition, evidence from the history, physical examination, or laboratory findings that the signs and symptoms are the direct physiological consequences of a medical condition or substance use. The other common features include hallucination, delusions, or inappropriate affective states. Haloperidol is the most frequent drugs with a dose of 0.25 to 0.5 mg every 4 hours for the elderly and 3mg once per day in healthy patients. In addition, 4.5 to 5.1 mg per day of olanzapine a nd 25–200 mg of quetiapine, which are atypical antipsychotics, have an enough evidence for the treatment of delirium while preventing EPSs from haloperidol [23].
FGAs also work as the acetylcholine muscarinic M1 antagonists, histamine H1 antagonists, and α1 adrenergic antagonists. All these actions on neurotransmitter receptors will evoke ADRs after treatment (Table 3, 4) [2425]. FGAs for these receptors present ADRs like dry mouth, constipation, sleepiness, weight gain, and orthostatic hypotension, which are similar to those of typical antidepressants.
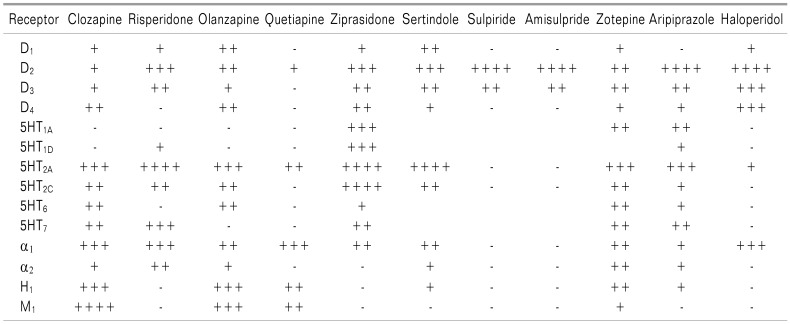
SGAs have fewer ADRs, such as EPSs, which distinguishes them from FGAs [234]. The SGAs also work on multiple neurotransmitter receptors, including not only the dopamine receptors, but also the serotonin (5-HT), histamine, adrenalin, and muscarinic acetylcholine receptors. Their affinity to each receptor is different, and the disparity among receptor affinity could explain the mechanism of action a nd a dverse r eactions of each d rug (Table 3, 4) [2425].
The representative characteristic mechanisms of action of the SGAs, compared to haloperidol (FGAs), are reduced D2 and increased 5-HT2A receptor binding. Compared to haloperidol, clozapine, quetiapine, olanzapine, and zotepine show a lower affinity to the D2 receptor; however, risperidone, ziprasidone, sertindole, clozapine, olanzapine, zotepine, and aripiprazole have a higher affinity to the 5HT2A receptor.
Long-term, the strong binding to the D2 receptor of FGAs produces two representative ADRs: EPSs and hyperprolactinemia. To avoid these ADRs, it is better to choose certain SGAs, such as clozapine, quetiapine, olanzapine, and zotepine, which produce short-term, weak binding to the receptor.
Regarding the 5HT2A receptor, which is related to sleep, certain SGAs, such as risperidone, ziprasidone, sertindole, clozapine, olanzapine, zotepine, aripiprazole, or quetiapine are preferred for the treatment of insomnia using the somnolescent effect of SGAs. On the contrary, sulpiride and amisulpride do not produce somnolence, like the FGA haloperidol (Table 4) [8242526].
Certain SGAs, such as, clozapine, olanzapine, quetiapine, and zotepine, which induce histamine H1 receptor blockade, may produce sedation and weight gain. On the contrary, risperidone, ziprasidone, sulpiride, and amisulpride, as well as haloperidol (FGA) do not produce sedation and weight g ain (Table 4) [8242526].
Alpha-1 adrenergic antagonism by SGAs produces hypotension, sedation, and weight gain. Clozapine, risperidone, quetiapine, (haloperidol), ziprasidone, sertindole, and zotepine may produce these ADRs.
Focused on weight gain due to SGAs, clozapine, olanzapine, and quetiapine significantly increase the body mass index (BMI), however, risperidone, sertindole, zotepine, amisulpride, aripiprazole, and ziprasidone were less likely t o increase BMI. Proposed mechanisms of weig ht gain by SGAs are related receptors (the H1, 5-HT2A, 5-HT2C, M3, and adrenergic receptors), reproductive hormones, other neurotransmitters, insulin, neuropeptides, cytokines, and g enetic factors [27282930].
SGAs, such as olanzapine and clozapine, rather than quetiapine and ziprasidone, have been frequently reported as causing the development of new-onset diabetes mellitus (DM) and impaired g lucose t olerance o r exacerbation o f existing type 1 and 2 DM. Supposed mechanisms include induction of peripheral insulin resistance and direct influence on pancreatic beta-cell function by 5-HT1A/2A/2C receptor antagonism, or inhibition of the alpha 2 adrenergic receptors. Known risk factors are a family history of DM, obesity, and Neg roid ethnicity [313233].
Anti-muscarinic effects due to M1 blockade, such as dry mouth, may e xhibit themselves during the a dministration of clozapine, olanzapine, quetiapine, and zotepine, in order of frequency. On the contrary, SGAs, such as risperidone, ziprasidone, sertindole, sulpiride, aripiprazole, as well as haloperidol (FGA), do not produce anticholinergic ADRs (Table 4) [8242526].
The response to antipsychotics may differ from patient to patient. Antipsychotic equivalent oral doses are usually used based on 100 mg of chlorpromazine (CPZ) daily, and the daily oral dose is similar to 2 (1–5) mg of haloperidol, 100 (30–150) mg of clozapine, and 2 (0.5–3) mg of risperidone. However, even though the equivalent dose of other second-generation antipsychotic (SGA) drugs are not precisely established, 75 mg/d of quetiapine, 5 mg/d of olanzapine, and 7.5 mg/d of aripiprazole are equivalent to 100 mg/d of CPZ [343536].
For 1 mg/d of olanzapine, the equivalent doses of antipsychotics were as follows; CPZ 38.88 mg/d, haloperidol 0.74 mg/d, clozapine 30.62 mg/d, quetiapine 32.27 mg/d, amisulpride 38.33 mg/d, aripiprazole 1.41 mg/d, asenapine 0.89 mg/d, risperidone 0.38 mg/d, sertindole 1.08 mg/d, ziprasidone 7.92 mg/d, and zotepine 13.24 mg/d [3738].
It is well-known that both components of pain, which are composed of emotional and sensory experience, should be treated simultaneously. Antipsychotics may be effective if patinets show the positive symptoms of psychosis, such as delusion, hallucination, disorganized thought and behavior, perception disturbance, and inappropriate emotion.
Traditionally, non-psychiatric pain physicians hesitate to use FGAs because of ADRs, such as EPSs. The FGAs show their action by tight and long standing D2 receptor binding blockade, however, the SGAs act by quick releasing, weak binding to the D2 receptor block and 5-H2A receptor blockade. It is very important to recognize the EPSs from the beginning of the treatment, and to be well-acquainted with the treatment of EPSs.
Treatments include 1–6 mg/d of benz (a) tropine for dystonia, anticholinergics or 100–400 mg/d of amantadine for pseudo-parkinsonism, 30–120 mg/d of propranolol for akathisia, and valbenazine (12.5–100 mg/d) for 6 weeks for tardive dyskinesia. The abnormal involuntary movement scale (AIMS) is helpful for early detection of drug-induced tardive dyskinesia. Therefore, SGAs are expected to be used without difficulty, even though the potential metabolic ADRs, such as weight g ain or DM, exist.
When choosing an SGA, it is wise to make the selection after considering their representative adverse reactions: 1) for reducing sedation, aripiprazole, iloperidone, lurasidone, paliperidone, risperidone, ziprasidone, asenapine, olanzapine, clozapine, and quetiapine would be better choices, in that order. However, for insomnia, using the adverse reaction of sedation, following the reverse order, from quetiapine, becomes a better practice, 2) for patients who worry about weight gain, the preference would be aripiprazole, lurasidone, ziprasidone, asenapine, iloperidone, paliperidone, risperidone, quetiapine, clozapine, and olanzapine, in descending order, and 3) for patients showing EPSs, clozapine, iloperidone, quetiapine, aripiprazole, asenapine, lurasidone, olanzapine, ziprasidone, paliperidone, and risperidone, in descending order, are preferred.
A drug is usually used for treating certain symptom and/or signs of various diseases, disorders, and syndromes. Antipsychotics can use not only for the treatment of psychosis, but also for pain patients with positive symptoms of psychosis. Therefore, SGAs are worth using for pain patients with the positive symptoms, such as delusion, hallucination, disorganized thought and behavior, perception disturbance, and inappropriate emotion, during or after controlling all sensory components, while reducing worries about EPSs.
Go to : 
ACKNOWLEDGEMENTS
This study was supported by a 2-year (from 2017 to 2019) research grant from Pusan National University (Sang - Wook Shin).
Go to : 
References
1. Pain terms: a list with definitions and notes on usage. Recommended by the IASP Subcommittee on Taxonomy. Pain. 1979; 6:249. PMID: 460932.
2. Stroup TS, Alves WM, Hamer RM, Lieberman JA. Clinical trials for antipsychotic drugs: design conventions, dilemmas and innovations. Nat Rev Drug Discov. 2006; 5:133–146. PMID: 16518380.

3. Targum SD. Treating psychotic symptoms in elderly patients. Prim Care Companion J Clin Psychiatry. 2001; 3:156–163. PMID: 15014599.

4. Leo RJ, Regno PD. Atypical antipsychotic use in the treatment of psychosis in primary care. Prim Care Companion J Clin Psychiatry. 2000; 2:194–204. PMID: 15014629.

5. Seidel S, Aigner M, Ossege M, Pernicka E, Wildner B, Sycha T. Antipsychotics for acute and chronic pain in adults. Cochrane Database Syst Rev. 2008; CD004844. PMID: 18843669.

6. Seidel S, Aigner M, Ossege M, Pernicka E, Wildner B, Sycha T. Antipsychotics for acute and chronic pain in adults. J Pain Symptom Manage. 2010; 39:768–778. PMID: 20226624.

7. Ramachandraiah CT, Subramaniam N, Tancer M. The story of antipsychotics: past and present. Indian J Psychiatry. 2009; 51:324–326. PMID: 20048463.

8. Worrel JA, Marken PA, Beckman SE, Ruehter VL. Atypical antipsychotic agents: a critical review. Am J Health Syst Pharm. 2000; 57:238–255. PMID: 10674777.

9. Gottfries CG, Green L. Flupenthixol decanoate--in treatment of out-patients. Acta Psychiatr Scand Suppl. 1974; 255:15–24. PMID: 4533707.
10. Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2003; 27:1081–1090. PMID: 14642968.

11. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009; 35:549–562. PMID: 19325164.

12. Yang AC, Tsai SJ. New targets for schizophrenia treatment beyond the dopamine hypothesis. Int J Mol Sci. 2017; 18:1689.

13. Inada T. Evaluation and diagnosis of drug-induced extrapyramidal symptoms: commentary on the DIEPSS and guide to i ts usage. Tokyo: Seiwa Shoten Publishers;1996.
14. Knol W, Keijsers CJ, Jansen PA, van Marum RJ. Systematic evaluation of rating scales for drug-induced parkinsonism and recommendations for future research. J Clin Psychopharmacol. 2010; 30:57–63. PMID: 20075649.

15. Chouinard G, Margolese HC. Manual for the Extrapyramidal Symptom Rating Scale (ESRS). Schizophr Res. 2005; 76:247–265. PMID: 15949657.

16. Holloman LC, Marder SR. Management of acute extrapyramidal effects induced by antipsychotic drugs. Am J Health Syst Pharm. 1997; 54:2461–2477. PMID: 9359953.

17. Albanese A, Bhatia K, Bressman SB, Delong MR, Fahn S, Fung VS, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013; 28:863–873. PMID: 23649720.

18. Skogseid IM. Dystonia--new advances in classification, genetics, pathophysiology and treatment. Acta Neurol Scand. 2014; 129:13–19. PMID: 23683163.

19. Kurlan R, Rabin ML. Pseudoparkinsonism: a review of a common nonparkinsonian hypokinetic movement disorder. Adv Parkinson Dis. 2013; 2:108–112.

20. Glazer WM, Morgenstern H, Doucette JT. Predicting the long-term risk of tardive dyskinesia in outpatients maintained on neuroleptic medications. J Clin Psychiatry. 1993; 54:133–139. PMID: 8098030.
21. Munetz MR, Schulz SC. Screening for tardive dyskinesia. J Clin Psychiatry. 1986; 47:75–77. PMID: 2867994.
22. Uhlyar S, Rey JA. Valbenazine (Ingrezza): the first FDA-approved treatment for tardive dyskinesia. P T. 2018; 43:328–331. PMID: 29896031.
23. Markowitz JD, Narasimhan M. Delirium and antipsychotics: a systematic review of epidemiology and somatic treatment options. Psychiatry (Edgmont). 2008; 5:29–36.
24. Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry. 2002; 47:27–38. PMID: 11873706.

25. Glazer WM. Extrapyramidal side effects, tardive dyskinesia, and the concept of atypicality. J Clin Psychiatry. 2000; 61:16–21.
26. Miller DD. Atypical antipsychotics: sleep, sedation, and efficacy. Prim Care Companion J Clin Psychiatry. 2004; 6:3–7.
27. Brixner DI, Said Q, Corey-Lisle PK, Tuomari AV, L'italien GJ, Stockdale W, et al. Naturalistic impact of second-generation antipsychotics on weight gain. Ann Pharmacother. 2006; 40:626–632. PMID: 16569802.

28. Gentile S. Contributing factors to weight gain during long-term treatment with second-generation antipsychotics. A systematic appraisal and clinical implications. Obes Rev. 2009; 10:527–542. PMID: 19460111.

29. Roerig JL, Steffen KJ, Mitchell JE. Atypical antipsychotic-induced weight gain: insights into mechanisms of action. CNS Drugs. 2011; 25:1035–1059. PMID: 22133326.
30. Taylor DM, McAskill R. Atypical antipsychotics and weight gain--a systematic review. Acta Psychiatr Scand. 2000; 101:416–432. PMID: 10868465.
31. Cohen D. Atypical antipsychotics and new onset diabetes mellitus. An overview of the literature. Pharmacopsychiatry. 2004; 37:1–11. PMID: 14750042.
32. Schwenkreis P, Assion HJ. Atypical antipsychotics and diabetes mellitus. World J Biol Psychiatry. 2004; 5:73–82. PMID: 15179666.

33. Haupt DW, Newcomer JW. Hyperglycemia and antipsychotic medications. J Clin Psychiatry. 2001; 62:15–26.
34. Leucht S, Samara M, Heres S, Patel MX, Woods SW, Davis JM. Dose equivalents for second-generation antipsychotics: the minimum effective dose method. Schizophr Bull. 2014; 40:314–326. PMID: 24493852.

35. Leucht S, Samara M, Heres S, Patel MX, Furukawa T, Cipriani A, et al. Dose equivalents for second-generation antipsychotic drugs: the classical mean dose method. Schizophr Bull. 2015; 41:1397–1402. PMID: 25841041.

36. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003; 64:663–667. PMID: 12823080.

37. Khouzam HR. Psychopharmacology of chronic pain: a focus on antidepressants and atypical antipsychotics. Postgrad Med. 2016; 128:323–330. PMID: 26821680.

38. Gardner DM, Baldessarini RJ, Waraich P. Modern antipsychotic drugs: a critical overview. CMAJ. 2005; 172:1703–1711. PMID: 15967975.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download