INTRODUCTION
Cardiac surgery can be performed through median sternotomy or thoracotomy. Acute post-sternotomy pain is a leading cause of post-cardiac chronic pain, especially if underrated, so adequate analgesia is a must to avoid such adverse effect and attain better patient satisfaction with enhanced rapid recovery [
1]. There are various modalities of pain management in such a group of patients including; local anesthetic infiltration, nerve block, nonsteroidal anti-inflammatory drugs (NSAIDs), opioids, dexmedetomidine, intrathecal and epidural analgesia, and multimodal analgesia. Multimodal combined analgesia seems to be the optimal approach for pain alleviation through the synergistic effects of variable analgesics and medications used with fewer side effects [
2].
Local anesthetics with adjuvants have been proved as an attractive option for postoperative pain alleviation and analgesics requirements reduction [
3456]. Many adjuvants have been described to augment the local anesthetic blockade and prolong its duration. Magnesium is one of the cheap and safe adjuvants which has an evident analgesic effect in conditions presenting with acute postoperative and chronic pain [
7]. Magnesium's effects are primarily based on its physiological calcium antagonism, where it prevents voltage-based calcium influx into the cell and has non-competitive antagonism on N-Methyl-D-aspartate (NMDA) receptors [
8]. The role of supplemental magnesium in providing perioperative analgesia is promising [
9].
The primary outcome of the present study was to evaluate the quality of pain alleviation during the first 48 postoperative hours through presternal infusion of a bupivacaine/magnesium mixture in comparison to either bupivacaine alone, or conventional intravenous analgesia. The secondary outcome was the implication of better pain control upon fentanyl consumption, extubation time, respiratory parameters, serum cortisol level changes, in addition to the incidence of chronic post-sternotomy pain.
Go to :

MATERIALS AND METHODS
This is a double-blind randomized controlled clinical trial, carried out between April 2017 and October 2017. It was approved by the local ethics committee of the Faculty of Medicine, Assiut University, Egypt and registered in the Clinical Trials with an identification number of NCT03106818.
Written informed consent was obtained from 90 adult patients scheduled for elective open heart surgery (valve replacement) with American Society of Anesthesiologist (ASA) physical status II or III. Exclusion criteria included; emergency surgery, previous sternotomy, impaired left ventricular systolic function (ejection fraction < 40%), preexisting neurologic or pulmonary dysfunction, significant hepatic or renal disease, allergy to local anesthetic, coagulopathy, prolonged cardiopulmnary bypass CPB > 120 minutes, postoperative application of an intra-aortic balloon pump, and major postoperative cardiac events e.g. malignant arrhythmia, myocardial infarction, severe hemodynamic instability requiring high inotropic support, and/or major postoperative bleeding which required re-exploration.
Patients were randomly allocated into one of three study groups using web-based generated random number tables (
http://www.randomizer.org). Allocation concealment was done using sealed opaque envelopes. Group 1 (n = 30) patients received a local infusion of bupivacaine with magnesium, Group 2 (n = 30) patients received a local infusion of bupivacaine only, and group 3 (n = 30) patients received a local infusion of normal saline (a placebo) with conventional intravenous analgesics.
All patients were prepared with a routine physical examination, electrocardiogram, echocardiography, and full laboratory investigations. They were trained to report pain on the visual analog scale (VAS), with VAS 0 meaning no pain, and VAS 10 the worst pain.
Anesthesia monitoring included five leads ECG, invasive blood pressure, pulse oximetry, capnography, oropharyngeal temperature, airway pressure, and urine output. General anesthesia was induced with fentanyl 5 µg/kg, midazolam 0.1 mg/kg, propofol 1–2 mg/kg, and lidocaine 1–2 mg/Kg. Cisatracurium in the dose of 0.1 mg/kg was administered to facilitate endotracheal intubation. Anesthesia was maintained using 1:1.5% isoflurane in an oxygen air mixture (1:1 ratio), in addition to continuous fentanyl infusion (1 µg/kg/hr). Cisatracurium was repeated during surgery as required to ensure proper muscle relaxation.
Surgical procedure was established by the same team in all patients through a median sternotomy incision. After obtaining activated clotting time ACT ≥ 400 seconds, the aorta and the vena cava were cannulated for the establishment the CPB. The procedure was conducted in mild hypothermia, and cardioplegic arrest was achieved with antegrade potassium cold cardioplegia.
By the end of the procedure, coagulation reversal and separation from the CPB were done. After sternal wiring, a small diameter multi-hole soft catheter used for epidural analgesia (Portex Epidural Catheter 18-gauge; Smith Medical ASD Inc., Keene, NH) was positioned anteriorly to the sternum above the fascia in the subcutaneous tissue and left for 48 hours postoperatively. Steri-strips (3M Neuss, Germany) and wound dressing were used to secure the catheter.
On the morning of surgery, an anesthetist who did not visit the patient opened a closed envelope to learn the randomization group and prepare the study drugs. A disposable 50 ml elastomeric unlabeled coded reservoir that delivers a continuous presternal infusion of the prepared solution at a fixed rate of 3 ml/h was prepared. Solution for intravenous injection in unlabeled coded 5 ml syringes was given every 8 hours, and 100 ml infusion bottles for intravenous were prepared as well, and given every 6 hours. Patients and the postoperative observers were blinded to the drugs and doses given.
Our groups were managed as following; (Group 1) the elastomeric reservoir contained bupivacaine 0.125% (1.25 mg/ml), and magnesium sulfate 5% (50 mg/ml). Saline was used in the 5 ml syringes and the 100 ml infusion bottles (Group 2) the elastomeric reservoir contained bupivacaine 0.125% (1.25 mg/ml) alone. Saline was used in the 5 ml syringes and the 100 ml infusion bottles, and (Group 3) the elastomeric reservoir contained normal saline. The 5 ml intravenous syringe contained 30 mg ketorolac promethazine, while the intravenous 100 ml infusion bottles contained 1000 mg paracetamol.
By the end of surgery, fentanyl infusion was stopped, and the patient was transferred intubated to the postoperative ICU and mechanically ventilated. Extubation was allowed with stable hemodynamics, adequate airway reflexes, normothermia, acceptable blood gas analysis, and normal electrolytes. A fentanyl shot(s) in a dose of 25 µg was used as rescue analgesia when the VAS > 3. Time to the first requirement of recuse analgesia and total fentanyl consumption over the postoperative 48 h were recorded.
Pain was assessed by an intensivist using the VAS scale as early as possible when the patient could reply and assign his or her VAS, then every 4 hours during the first 12 hours, and every 6 hours over the next 36 hours. The patient was asked to take a deep breath and the intensity of pain was recorded. Hemodynamics (mean arterial blood pressure [MAP] and heart rate [HR]) during the first operative day were recorded as well. The quality of oxygenation was assessed by arterial blood gas (ABG) analysis at the time of induction, general anesthesia, extubation time, then at the 6th, 12th, and 24th hours. Calculation of the corresponding PaO2/FiO2 ratios were obtained. Regarding the use of magnesium infusion, it is routine in our institute to evaluate our patients' knee tendon jerk, respiratory rate, and urine output to be kept > 0.5 ml/kg/h, and in case of any clinical suspicion of toxicity, serum magnesium was measured.
The blood samples for serum cortisol level monitoring were collected in the evenings of the 1st and 2nd postoperative days. The samples were collected into gel tubes, then the blood samples were allowed to sit for 15 minutes for clotting, and centrifuged at 5000 rpm for 6 minutes. The serum samples were transferred to 1.5 ml polypropylene tubes and stored at −80 degrees centigrade until the analysis. Plasma cortisol was measured by a commercial immunoassay (Modular Analytics E170, Roche Diagnostics, Mannheim, Germany). The analytical sensitivity was 8.5 nmol/l; the intra-assay coefficient of variation was 1.7% for cortisol at 129 nmol/l. The inter-assay coefficient of variation was 4.7% for cortisol at 102 nmol/l and 2% for cortisol at 940 nmol/l.
The occurrence of any postoperative complication, such as sternal wound infection or delayed healing during the first postoperative two weeks, was recorded. Pain assessment for the occurrence of chronic post-sternotomy pain was done by one of the investigators who was not aware of the group randomization using the VAS scale after two months following surgery.
Statistical analysis
A calculated sample size of 28 patients would have 80% power to detect a difference of 20% in a postoperative pain score with a type I error of α = 0.05 using a confidence interval of 95%. Thirty patients were enrolled in each group to compensate for any dropouts during the study.
Data were expressed as mean, standard deviation (SD) or standard error (SE), numbers, and/or ratio, as appropriate. Categorical variables were compared using a chisquare (χ2) test. Continuous variables were compared using the one-way analysis of variance (ANOVA) test. Nominal and non-normally distributed variables were compared using the Kruskal-Wallis H test.
Statistical analysis was done using the computer program IBM SPSS Statistics version 22. A P value < 0.05 was considered statistically significant.
Go to :

RESULTS
A total number of 90 patients were enrolled in this study as shown in the CONSORT flow diagram (
Fig. 1). They were comparable in age, gender, body mass index, ejection fraction, CPB time, duration of surgery, and ICU stay, with insignificant differences (
Table 1).
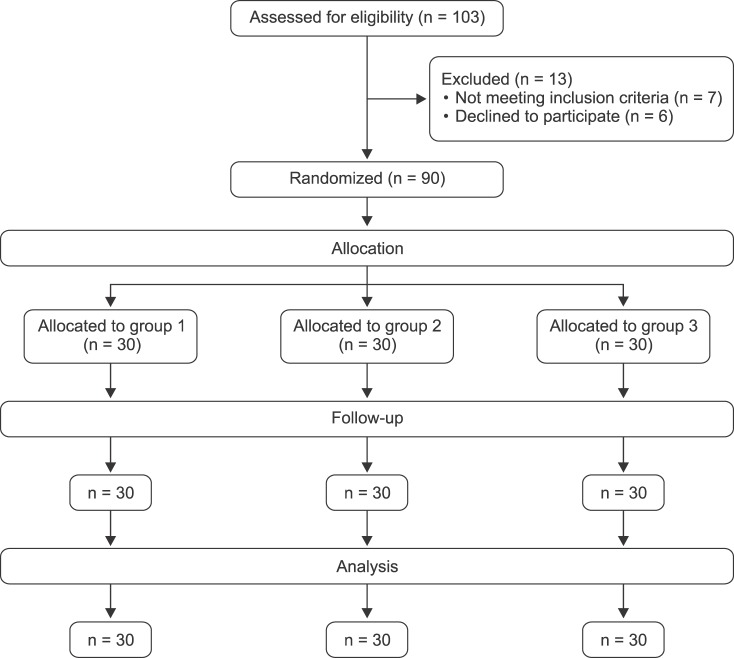 | Fig. 1CONSORT flow diagram.
|
Table 1
Patients, Surgical Characteristics Postoperative Clinical Data
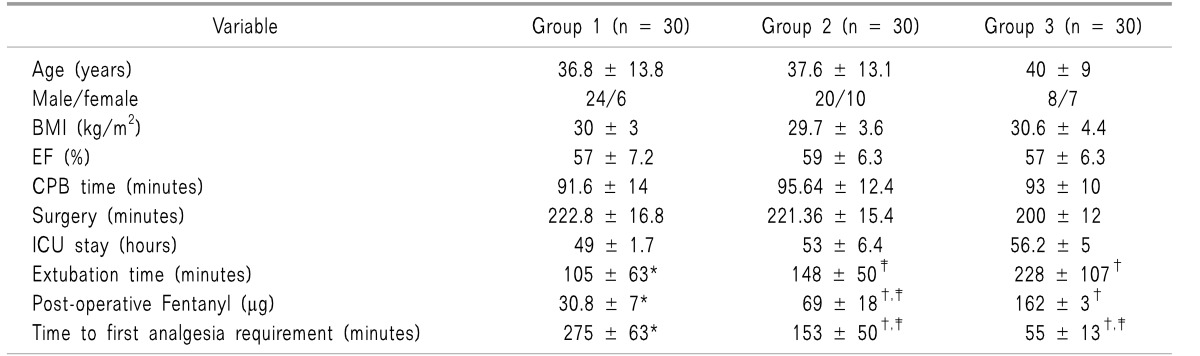

The mean VAS was significantly lower in group 1 than the other two groups during the whole study period (
Fig. 2).
 | Fig. 2Visual analogue scale (VAS) of the three studied groups at different. Group 1: bupivacaine with magnesium group, Group 2: bupivacaine group, Group 3: conventional intravenous analgesics group. *P < 0.05 was considered statistically significant between the three studied groups.
|
Time to first rescue analgesia showed a significant difference among the three groups, with the longest time in group 1, followed by group 2 and group 3, as shown in
Table 1. Fentanyl consumption was significantly lower in group 1 than in groups 2 and 3 (30.8 ±7 µg vs. 69 ± 18 µg, and 162 ± 3, respectively) as also shown in
Table 1. The incidence of chronic pain in the three groups varied, but with insignificant differences between the three groups (
Fig. 3).
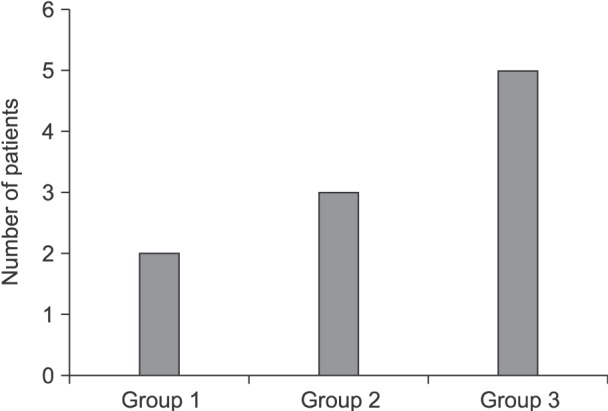 | Fig. 3Incidence of chronic pain in the three groups after two months follow up. Group 1: bupivacaine with magnesium group, Group 2: bupivacaine group, Group 3: conventional intravenous analgesics group. Data are presented as number.
|
Cortisol levels (
Fig. 4) were higher than the normal value in all groups during the 1
st postoperative day with insignificant difference between the three groups. The 2
nd day, cortisol level values significantly decreased toward a normal level (12 ± 3.03 µg/dl) in comparison to the 1
st day value in group 1 only.
 | Fig. 4Cortisol level of the three studied groups. Group 1: bupivacaine with magnesium group, Group 2: bupivacaine group, Group 3: conventional intravenous analgesics group. Data are presented as mean ± SE.
|
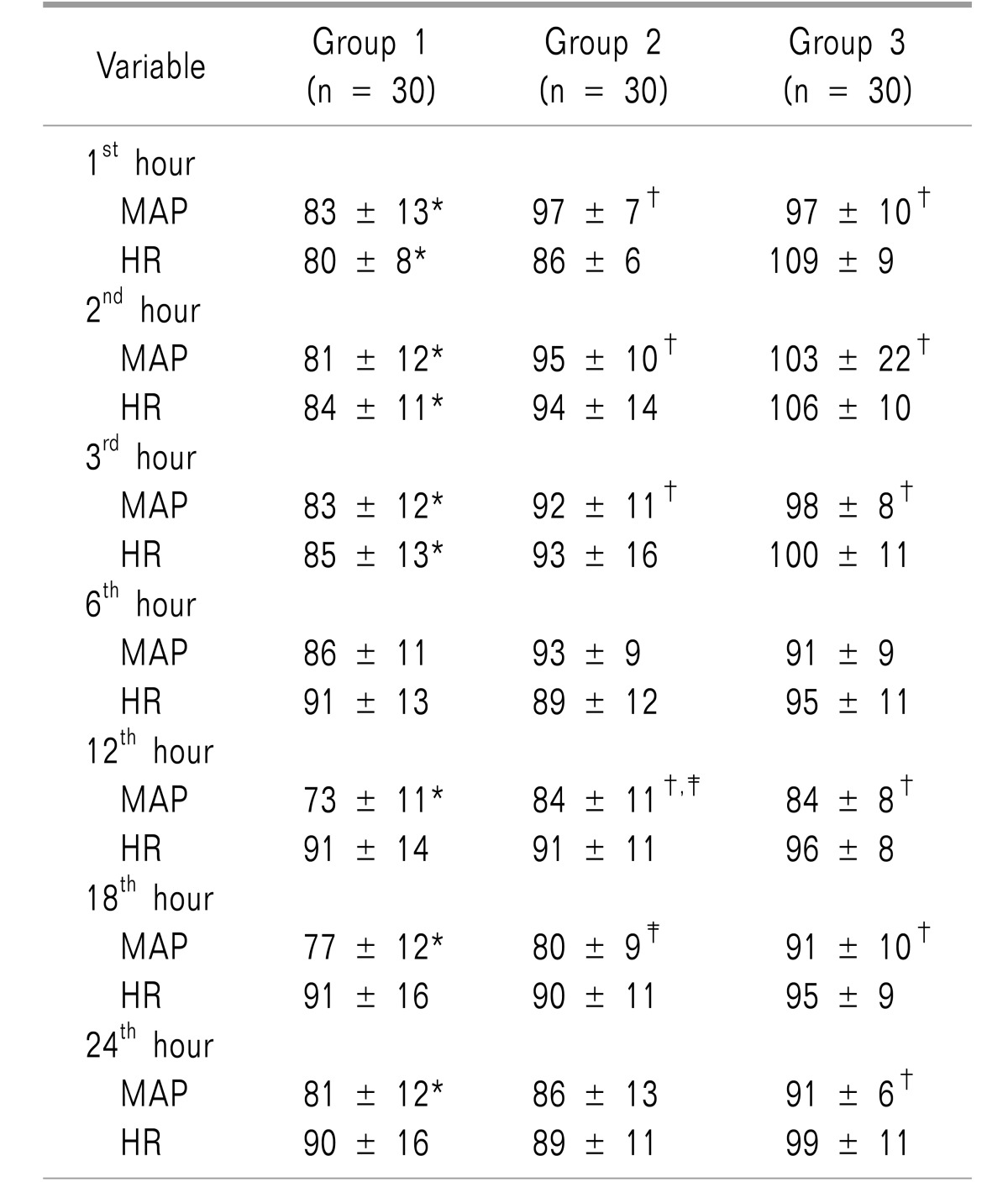
Table 2 shows the hemodynamic changes. MAP showed significant differences between the study groups most of the day except at the 6
th hour, with the lowest values in group 1. Heart rate as well, showed significant differences between the three groups only during the first three postoperative hours.
Table 2
Postoperative Mean Arterial Pressure (MAP) and Heart Rate (HR) Follow-up

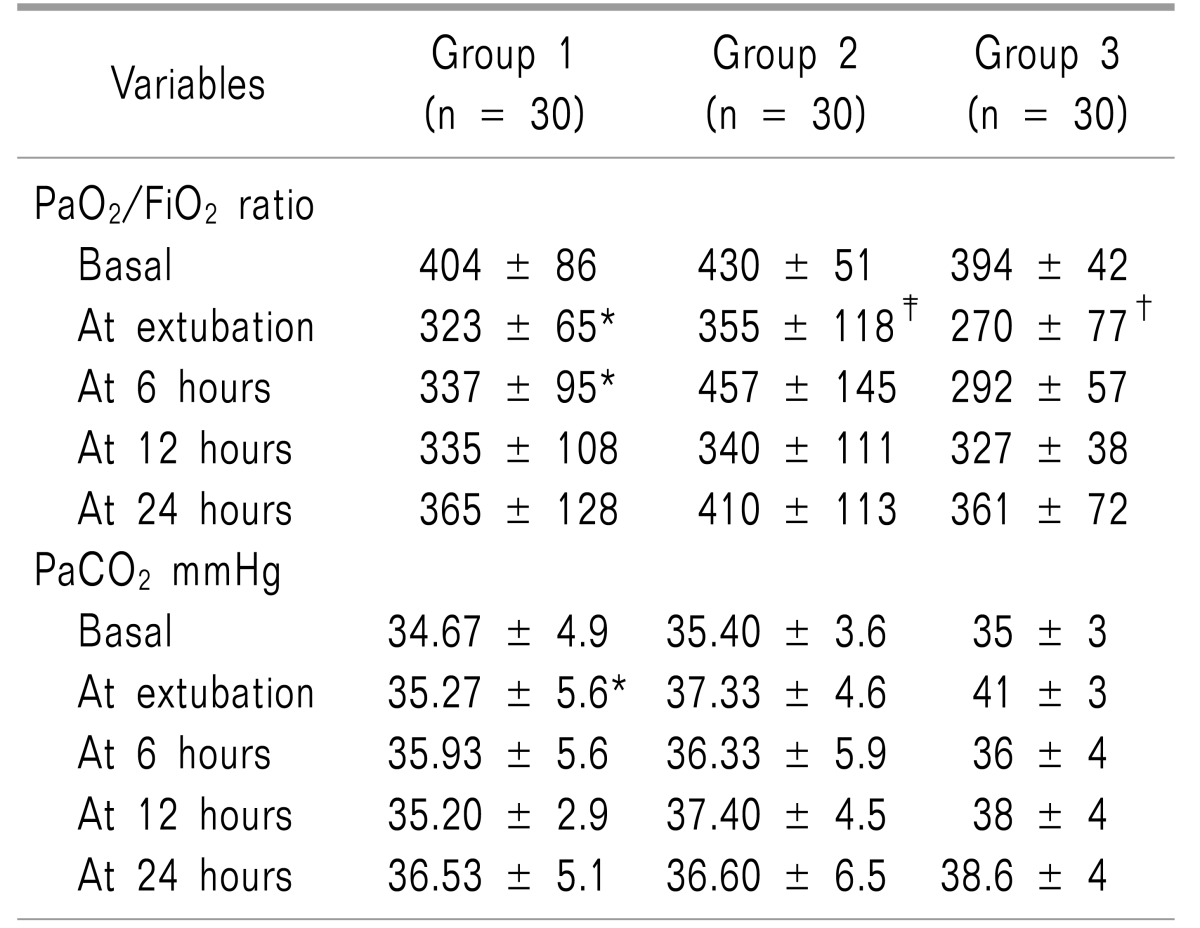
Respiratory parameters are demonstrated in
Table 3. The PaO
2/FiO
2 ratio showed significant differences between the three groups along the first six postoperative hours and the PaO
2/FiO
2 ratio was significantly higher in groups 1 and group 2 in comparison to group 3. In regard to PaCO
2, it showed significant difference between the three groups at extubation time only. Time to extubation was the shortest in group 1, in comparison to group 2, and was significantly longest in group 3 (
Table 1).
Table 3
Follow-up of PaO2/FiO2 Ratio, PaCO2 and pH

There were no local wound complications within the follow-up period.
Go to :

DISCUSSION
Our study has demonstrated a satisfactorily good quality of pain alleviation with the use of presternal injection of bupivacaine/magnesium infusion when compared to bupivacaine alone, or the conventional intravenous analgesia. The VAS, and fentanyl consumption were significantly higher in the conventional intravenous analgesia group. Optimal analgesia in the bupivacaine/magnesium group can be assumed to be the well-known analgesic efficacy of magnesium.
The use of magnesium as an additive to local anesthetic is not a new modality. Many studies have demonstrated its effectiveness in pain alleviation through local wound infiltration either alone or with local anesthetics [
1011]. Interestingly, we think that the benefit here is not limited to its antinociceptive effect, but also in a reduction of the dose of bupivacaine used; hence, avoiding its toxicity especially after such major surgery affecting the pharmacokinetics of local anesthetics [
1213].
These results are in agreement with Chiu and his colleagues who have found that continuous bupivacaine 0.15% infusion through a locally embedded catheter during the wound closure of minimally invasive cardiac surgeries has reduced the analgesia requirement and provided an excellent way of continuous analgesia. They considered the possibility that this modality of analgesia could be superior to patient-controlled analgesia [
14]. Our results also were in agreement with Nasr et al. [
15], who have demonstrated the same effect, but they used a higher concentration of bupivacaine (0.25%) alone. In their study bupivacaine was infused at a rate of 2 ml/h via the same technique we used, and they found a signficant reduction in the postoperative morphine requirement, while the level of bupivacaine in the blood did not exceed a toxic level [
15].
Kocabas et al. [
16] highlighted levo-bupivacaine 0.25% infiltration of the median sternotomy incision and the mediastinal tube insertion sites in addition to patient-controlled morphine analgesia for optimal pain alleviation. They found somewhat similar results regarding postoperative morphine consumption, which was reduced when compared with patient-controlled morphine analgesia alone during the initial 24 hours after cardiac surgery [
16].
We followed-up the participants after two months, inspecting the incidence of chronic post-sternotomy pain. A few patients have developed this type of pain with the difference between the groups being insignificant. Lee et al. [
17] have demonstrated that perioperative administration of epidural magnesium in patients undergoing video-assisted thoracic surgery did not make any significant difference in chronic postoperative pain over three months when compared to a placebo group. Two other recent studies, which assessed the implication of the perioperative use of systemic magnesium sulfate on the chronic postoperative pain found that magnesium administration has not demonstrated any significant effect on the incidence of chronic pain in the postoperative period [
1819].
Cortisol, as one of the stress biomarkers, increases in many situations reflecting pain, fatigue, and sleep disturbance [
2021]. We have studied cortisol postoperative serum level changes in the 1
st and 2
nd postoperative days, and found that the first postoperative evening values of cortisol increased in all participants. On the evening of the 2
nd post-operative day, serum cortisol had signficantly returned back to its normal value only in the bupivacaine/magnesium group. We assume that better pain alleviation and stress, secondary to the use of magnesium, could result in such normalization of the serum cortisol level. The correlation between pain and the serum cortisol level has been described in multiple studies. Gottardo et al. evaluated the implications of variable pain management modalities after surgical castration in piglets on serum cortisol, and they found that cortisol levels were significantly higher in the group which did not receive analgesia [
22]. Another clinical trial showed that optimal pain control through epidural anesthesia and analgesia has suppressive effects on the intraoperative and postoperative stress response together with reduced postoperative pain scores, and hence the lower blood glucose and cortisol levels when compared to fentanyl postoperative analgesia alone [
23].
MAP and HR showed lower values preferentially in the bupivacaine with magnesium group, and this could have resulted from better pain control as well as the direct effect of magnesium. It is well established that regional magnesium can attenuate stress mediated hemodynamic stimulation for greater stabilization of the heart rate and blood pressure. These results were in agreement with Kamble et al. [
24] who found better control and attenuation of hemodynamic responses when they used an intra-peritoneal magnesium injection before laparoscopic cholecystectomy. This result may also be assumed to come from the ability of magnesium to decrease the release of catecholamines from nerve endings as well as the adrenal medulla [
25]. Magnesium can produce vasodilatation and the attenuation of vasopressin upon the vascular tone [
26]. Another two studies have documented the hemodynamic stability which was offered by local infiltration of magnesium through the attenuation of stress response [
1027].
Extubation times in our study groups where local infiltration was done, were significantly shorter than that of the conventtional intravenous analgesia group, while at the same time the difference in the total hours of their ICU stay was not significant. Significantly higher and better values in the PaO2/FiO2 ratio were found in the local infiltration groups than the conventional group 3 in the first six hours after discontinuation of mechanical ventilation and extubation. The significant difference between groups regarding PaCO2 was noticed only at the time of extubation; however, all changes were within accepted physiological ranges.
These findings were in agreement with Nasr et al. [
15] who found a shorter extubation time, better respiratory parameters, (PaO
2/FiO
2, PaCO
2, and pH) in the group which received local bupivacaine wound infiltration after cardiac surgery. Due to our local postoperative ICU protocols, we found a non-significant difference between the groups regarding their ICU stay, yet it was shorter in group 1. In another study, the length of hospital stay was significantly reduced in a group of open heart surgery patients, who postoperatively received continuous bupivacaine 0.5% local wound infusion at a rate of 4 ml/h, and showed better pain control, patient satisfaction, and hence they were able to ambulate earlier [
28].
No complication related to the procedure we used has been recorded during the follow-up period. The same was found in the previously mentioned studies [
151928]. However, a study done by Agarwal et al. [
29] demonstrated a high incidence of sternal wound infection with increased incidence of catheter-related problems, which led to discontinuation of the trial. An increased wound infection could be consistent with a theoretical concern that local anesthetics could offer anti-inflammatory effects. Local anesthetics exert such anti-inflammatory effects through their ability to diminish the release of inflammatory mediators and granulocyte activity which may lead to immunosuppression, and that is more likely to occur with levorotatory stereoisomer ropivacaine, and its closely related chiral congener levobupivacaine, than with racemic anesthetics like bupivacaine [
3031]. On the other hand, Waite et al. have concluded that although lidocaine and bupivacaine influence local inflammatory and proteolytic factors, they did not impair the rate of healing in either of two well-established models (mimicking normal human wound healing and impaired age-related healing) [
32].
We did not measure serum levels of bupivacaine or magnesium. There is also a need to study the implication of the technique upon chronic post-sternotomy pain over a larger number of patients.
This study denoted that magnesium as an additive to bupivacaine local infiltration after cardiac surgery has offered a better postoperative pain control, less opioid consumption, and earlier discontinuation of mechanical ventilation. Obviously the technique has no evident effect on chronic post-sternotomy pain.
Go to :








 PDF
PDF Citation
Citation Print
Print






 XML Download
XML Download