Abstract
We present a patient with metastatic colon carcinoma who developed paraplegia following a neurolytic splanchnic block. A 41-year old man with metastatic adenocarcinoma of the colon received a splanchnic neurolytic block using alcohol because of severe abdominal pain. Bilateral motor weakness and a sensorial deficit in both legs developed after the procedure. Diffusion magnetic resonance imaging revealed spinal cord ischemia between T8 and L1. The motor and sensorial deficits were almost completely resolved at the end of the third month. We think that anterior spinal artery syndrome due to reversible spasms of the lumbar radicular arteries using alcohol have resulted in transient paraplegia. The retrograde spread of alcohol to neural structures may have also contributed.
Go to : 
The World Health Organization (WHO) analgesic ladder has been proposed to provide optimal pain relief in 70–80% of cancer patients by using pharmacological treatment [1]. Inadequate pain control despite the use of the ladder, doses that were poorly tolerated due to side effects or unpredictable dose-response relation may sometimes lead to difficulty in the institution of effective pain management.
The efficacy of the thoracic splanchnic nerve and celiac ganglion neurolytic blocks have been established for the control of pain in upper gastrointestinal tumors in various studies [2345]. They provide superior pain relief with a changing incidence of side effects like orthostatic hypotension, diarrhea, back pain, retroperitoneal hemorrhage, or neurological injury [6].
We present a patient with metastatic colon carcinoma who developed paraplegia following a neurolytic splanchnic block performed to relieve abdominal pain.
Go to : 
A 41-year old man with a diagnosis of metastatic adenocarcinoma of the colon was admitted to the Pain Unit because of severe abdominal pain. He had a progressively increasing epigastric pain radiating towards his back for two months. He was using tramadol 300 mg/day and non-steroidal anti-inflammatory drugs which no longer provided satisfactory analgesia. He was also prescribed transdermal fentanyl 25 µg/h, but did not use regularly because of side effects.
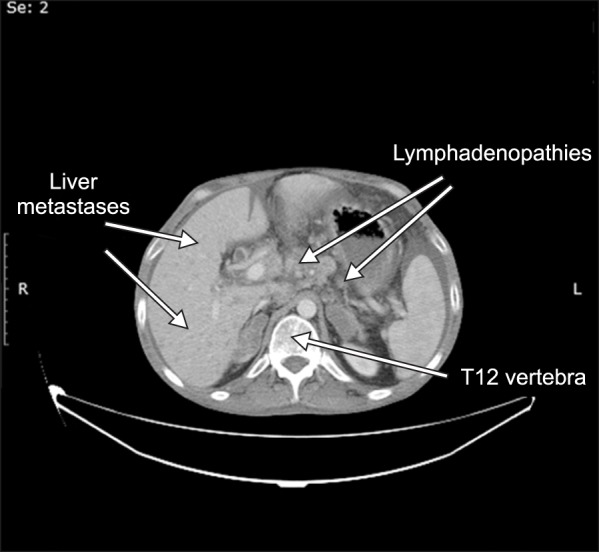
After evaluation, it was learnt that he had undergone surgery 4 years before and had colostomy. He had chemotherapy for a long time but he was now regarded as chemotherapy resistant and no further treatment was planned. A recent computerized tomography revealed multiple metastatic lesions in the liver and enlarged peri-pancreatic, peri-aortic, peri-caval and hepatogastric lymph nodes tending to conglomerate (Fig. 1).
He was subsequently hospitalized in Pain and Palliative Care Unit for pain management. A splanchnic neurolytic block was planned and written informed consent was obtained from the patient. The procedure was performed under fluoroscopic guidance in the operating room. He was placed in the prone position with a pillow placed under the abdomen. After establishment of a diagnostic splanchnic block with a local anesthetic mixture of 5 ml of lidocaine 2% and 5 ml of bupivacaine 0.5% using retro-crural technique and obtaining pain relief without motor weakness, a bilateral neurolytic splanchnic block with 10 milliliters of alcohol on both sides was performed. The place of the needles and spread of the solution was confirmed with a contrast medium.
While administering alcohol, sedation with propofol was given to prevent the feeling of pain and movement during injection. He was turned to supine after cleaning and dressing of the injection site of the skin.
When he was transferred to the pain and palliative care service back, it was noticed that he had bilateral motor weakness and sensorial deficit in both legs. On consultation with neurologist, intravenous steroid, oral vitamin B and anti-aggregates were started.
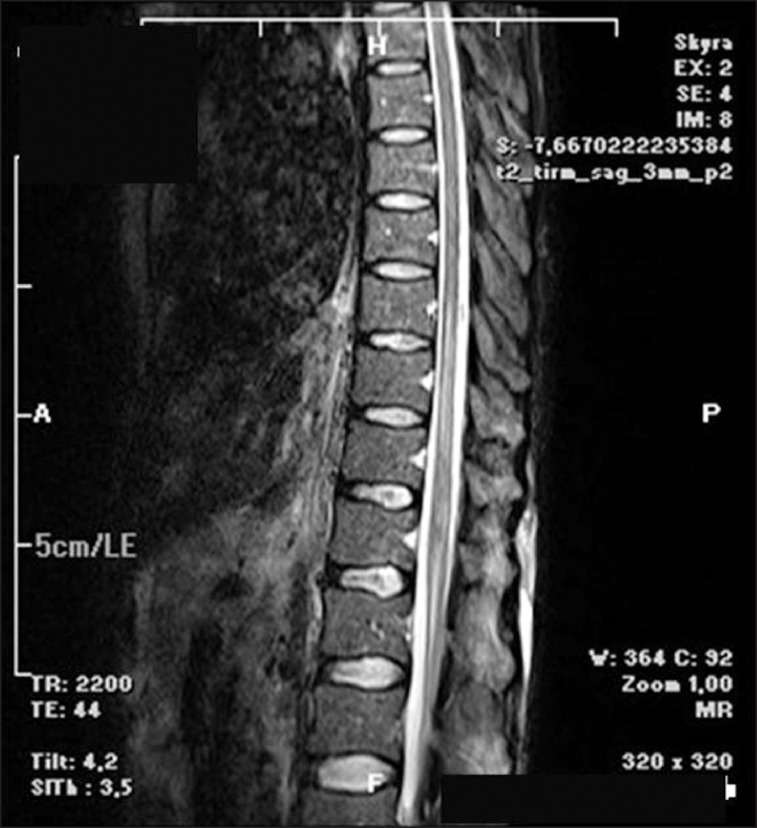
The motor block began to improve the next day. The motor strength was assessed as 4/5 in the right leg, 3/5 in the left, and there was patchy sensorial deficit in both proximal part of the legs. The sensorial examination was normal in other dermatomes. The thoracolumbar diffusion magnetic resonance imaging revealed spinal cord ischemia between the corpuses of vertebra T8 and L1 (Fig. 2).
He had no epigastric or back pain due to his cancer anymore and was integrated into a physiotherapy program. He was able to walk with a walker one month later, but had still a bladder catheter due to neurogenic bladder which was changed to clean intermittent catheterization at the second month. The motor and sensorial deficits were almost completely resolved at the end of the third month. The patient died because of tumor progression and metastases at the fourth month at home.
Go to : 
The WHO analgesic ladder is a framework used to guide the worldwide pharmacologic treatment of pain in chronic pain and palliative care patients [7]. It advocates the stepwise safe use of opioids for moderate to severe pain due to cancer. When it is difficult to make dose escalation due to side effects or the pharmacological treatment proves to be ineffective, invasive procedures must be taken into account to optimize pain relief [8].
In our patient, we performed splanchnic nerve neurolysis to manage pain because of his colonic cancer. Despite the use of a test block with local anesthetic and the use of contrast medium to verify the drug spread, he developed paraplegia following the neurolytic block.
The most serious and feared complications of splanchnic nerve and celiac plexus blocks are neurologic [9]. There are several mechanisms for the occurrence of neurologic deficit. Drug misplacement and anomalous or excessive retro-crural spread can affect epidural and lumbar somatic nerve roots. Unintended intrathecal injection of neurolytic agents can result in permanent paraplegia. Direct injection of the alcohol solution into subarachnoid or epidural spaces was considered unlikely in the present case because of limited spread of paralysis.
The clinical findings of our patient suggest ischemia of the anterior spinal cord with complete motor and sensory paralysis. The vascular supply of the spinal cord was originally described by Adamkiewicz in 1882. There is great variability among the radicular arteries and the largest is typically called the artery of Adamkiewicz which may arise from T7 to L4. Lying on the left in 80% of cases, it enters via a single intervertebral foramen to supply the anterior spinal artery of the lower two-thirds of the cord [10].
The neurological complications may occur due to a spasm of the lumbar segmental arteries that perfuse the spinal cord. The possible mechanisms of anterior spinal artery syndrome can be either mechanical damage to the artery by a needle or chemical by the neurolytic drug [11]. Arterial vasospasm in response to local anesthetic or alcohol, direct needle trauma, extramural arterial compression due to limited spread of the solution in a confined space with the presence of enlarged lymph nodes, retrograde diffusion of alcohol to the neural structures are all suspected etiologies in our patient.
We have performed the splanchnic block under fluoroscopic guidance and the needle position and spread of the drug was confirmed by the use of contrast medium. The aspiration was also negative for blood before injection of the alcohol. The patient had complete pain relief for weeks after the block suggesting the correct placement of the neurolysis. Therefore, direct intra-arterial injection does not seem to be possible. Although radiologically guided techniques minimize the incidence of direct intravascular injection, it is known that neurolytic drugs deposited peri-vascularly may alter arterial reactivity and cause vasospasm. We have used propofol for sedation during the injection of alcohol to prevent pain and patient movement. This may also have masked the immediate detection of neurological injury.
There are several reports of paraplegia following neurolytic celiac plexus blocks using phenol or alcohol. Eisenberg et al. reported the incidence to be between 0.1% and 0.5% in their meta-analysis [12]. Davies et al. have reported 4 cases of paraplegia among 2730 patients in a 5-year period, in all of whom alcohol was injected under fluoroscopic control [13]. Jabbal and Hunton [14] described a case of reversible paraplegia following a celiac plexus block. The patient was able to walk with a stick within a week of the event. Similar cases with different levels of paresthesia and different recovery times were reported in other studies [1516].
In the present case, we think that anterior spinal artery syndrome due to a reversible spasm of the lumbar radicular arteries caused by the alcohol have resulted in transient paraplegia. The retrograde spread of alcohol to neural structures may have also contributed to this complication. Radiologic imaging, performance of the procedure by experienced physicians, appropriate indications, as well as detailed information and informed consent from the patients are necessary.
Go to : 
References
1. Vargas-Schaffer G. Is the WHO analgesic ladder still valid? Twenty-four years of experience. Can Fam Physician. 2010; 56:514–517. PMID: 20547511.
2. Mercadante S, Catala E, Arcuri E, Casuccio A. Celiac plexus block for pancreatic cancer pain: factors influencing pain, symptoms and quality of life. J Pain Symptom Manage. 2003; 26:1140–1147. PMID: 14654266.

3. Süleyman Ozyalçin N, Talu GK, Camlica H, Erdine S. Efficacy of coeliac plexus and splanchnic nerve blockades in body and tail located pancreatic cancer pain. Eur J Pain. 2004; 8:539–545. PMID: 15531222.

4. Papadopoulos D, Kostopanagiotou G, Batistaki C. Bilateral thoracic splanchnic nerve radiofrequency thermocoagulation for the management of end-stage pancreatic abdominal cancer pain. Pain Physician. 2013; 16:125–133. PMID: 23511679.
5. Shwita AH, Amr YM, Okab MI. Comparative study of the effects of the retrocrural celiac plexus block versus splanchnic nerve block, C-arm guided, for upper gastrointestinal tract tumors on pain relief and the quality of life at a six-month follow up. Korean J Pain. 2015; 28:22–31. PMID: 25589943.

6. Sehgal S, Ghaleb A. Neurolytic celiac plexus block for pancreatic cancer pain: a review of literature. Indian J Pain. 2013; 27:121–131.

7. World Health Organization. WHO's cancer pain ladder for adults [Internet]. Geneva: World Health Organization;cited 2017 Jun 22. Available at http://www.who.int/cancer/palliative/painladder/en/.
8. Stefaniak T, Basinski A, Vingerhoets A, Makarewicz W, Connor S, Kaska L, et al. A comparison of two invasive techniques in the management of intractable pain due to inoperable pancreatic cancer: neurolytic celiac plexus block and videothoracoscopic splanchnicectomy. Eur J Surg Oncol. 2005; 31:768–773. PMID: 15923103.

9. De Conno F, Caraceni A, Aldrighetti L, Magnani G, Ferla G, Comi G, et al. Paraplegia following coeliac plexus block. Pain. 1993; 55:383–385. PMID: 8121700.

10. Dommisse GF. The arteries, arterioles, and capillaries of the spinal cord. Surgical guidelines in the prevention of postoperative paraplegia. Ann R Coll Surg Engl. 1980; 62:369–376. PMID: 7436294.
11. Bowen Wright RM. Precautions against injection into the spinal artery during coeliac plexus block. Anaesthesia. 1990; 45:247–248. PMID: 2334038.

12. Eisenberg E, Carr DB, Chalmers TC. Neurolytic celiac plexus block for treatment of cancer pain: a meta-analysis. Anesth Analg. 1995; 80:290–295. PMID: 7818115.
13. Davies DD. Incidence of major complications of neurolytic coeliac plexus block. J R Soc Med. 1993; 86:264–266. PMID: 8505748.

14. Jabbal SS, Hunton J. Reversible paraplegia following coeliac plexus block. Anaesthesia. 1992; 47:857–858. PMID: 1280001.

15. van Dongen RT, Crul BJ. Paraplegia following coeliac plexus block. Anaesthesia. 1991; 46:862–863. PMID: 1952003.

16. Wong GY, Brown DL. Transient paraplegia following alcohol celiac plexus block. Reg Anesth. 1995; 20:352–355. PMID: 7577786.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download