INTRODUCTION
Pain has an immunosuppressive effect [
1]. Indeed, both acute and chronic pain could produce important changes in the sympathetic nervous system and induce hormonal imbalances in the hypothalamus, pituitary, adrenal, and gonad axis [
234]. These changes are associated with immune dysfunction, including natural killer (NK) cell function [
56].
NK cells are cytotoxic lymphocytes and critical components of innate immunity. Human NK cells exert direct cytotoxicity and secrete immunoregulatory cytokines and chemokines. NK cells comprise approximately 10–20% of the lymphocytes in human peripheral blood, and rapidly eliminate virally-infected, stressed and malignant cells [
7]. The involvement of NK cells in various disease conditions has been recognized. People with cancer, infection, or autoimmune disease show changes in NK cell-related immunity including subtype populations and cytotoxic activity compared with healthy individuals [
89].
NK cell activity against K562 tumor cells is reportedly increased by acute electrical and painful stimulation, but is blocked by local anesthesia before the stimulation [
10]. The overall effect of reducing pain by opioid medications on NK cells is unclear, but acute postoperative pain is itself immunosuppressive, as evidenced by the surgical stress response and increased glucocorticoid levels [
11]. Few studies have investigated changes in NK cell activity or subtype populations in patients with fibromyalgia, complex regional pain syndrome (CRPS), or back pain [
1213141516]. However, the above studies are not sufficient to reach any conclusions, which is likely to be due to the broad range of pain intensities and durations, and a lack of assessment of NK cell subtype populations or cytotoxic activity. The results are also conflicting, but some data suggest that the cytotoxic function and subpopulations of NK cells in chronic pain patients could be affected by pain intensity, or a specific disease such as CRPS [
1215].
The hypothesis of this study was that patients with chronic moderate-to-severe pain have different peripheral blood NK cell populations and activities than those of individuals with no pain.
Go to :

MATERIALS AND METHODS
The study protocol was approved by the Institutional Review Board of Chonnam National University Hospital (CNUH-2014-044). The inclusion criteria were 1) pain duration of ≥ 12 months and 2) a numerical rating scale (NRS) pain intensity score of > 4 (chronic, moderate-to-severe pain). Age-matched pain-free patients undergoing elective surgery for non-systemic, non-inflammatory, and non-malignant disease (e.g., vocal polyp, incidental unruptured cerebral aneurysm, or cosmetic oromaxillofacial surgery) were recruited as controls. Samples were obtained following acquisition of written informed consent from the participants. In total, 60 participants were thus assigned to the pain (P) or no pain (NoP) group. Sampling of peripheral venous blood was performed in the morning and preoperatively.
NK cell subset analysis was performed by fluorescence-activated cell sorting (FACS), a type of flow cytometry. Blood samples were collected in ethylenediaminetetraacetic acid tubes, and whole blood (100 µl) was treated with the following antibodies: peridinin chlorophyll protein complex-anti-CD45 (lymphocyte), Pacific blue anti-CD3 (T-lymphocyte), fluorescein isothiocyanate (FITC)-conjugated anti-CD19 (B-lymphocyte), FITC-anti-CD14 (monocyte), allophycocyanin-anti-CD56 (NK cell), and anti-CD16-PC7 (NK cell). Samples were incubated for 15 m at room temperature in the dark. Red blood cells were lysed by adding 2 ml of FACS lysing solution (BD Biosciences, San Jose, CA) and the samples were washed with phosphate-buffered saline (PBS). The remaining cells were re-suspended in PBS and analyzed for NK cell subsets.
Multicolor FACS analysis was performed on the harvested blood cells. In the lymphocyte collection gate, 20,000–50,000 events were analyzed by Kaluza software (Beckman Colter, Brea, CA). After selecting a bright CD45 event (
Fig. 1A), the CD3
−/CD14
−/CD19
− population was gated (
Fig. 1B) and evaluated for the expression of CD16 and CD56 surface receptors (
Fig. 1C). CD16-FITC vs.CD56-PC5 plots were used to distinguish following five NK subsets: 1) CD56
bright/CD16
− 2) CD56
bright/CD16
+ 3) CD56
dim/CD16
− 4) CD56
dim/CD16
+, and 5) CD56
−/CD16
+.
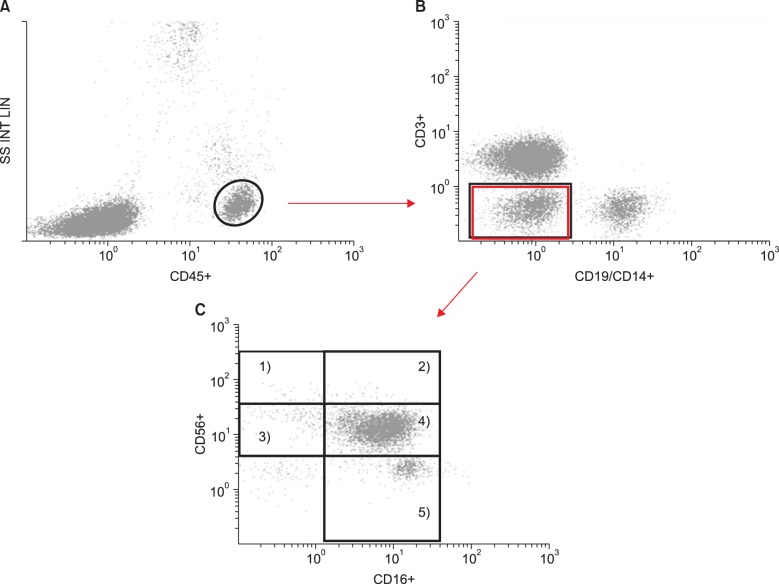 | Fig. 1Gating techniques used to identify NK subsets in whole blood samples. Each dot indicates a single cell analyzed by flow cytometry. As each cell is forced to pass through the laser beam, the cell is interrogated by the laser and scatters the light. Several detectors measure the light scattered from cells as either forward scatter (FS) or side scatter (SS); these measures are correlated with the size and granularity, respectively. The y-axis in panel (A) represents the amount of lights detected as SS, which is expressed using a linear scale. The intensity of the fluorescence obtained from cells stained with fluorescent antibodies is expressed using a logarithmic scale on the x- or y-axis of panel (A)–(C). After the lymphocytes were gated (A), NK cell subsets (CD3−/CD56+) were sequentially gated on the basis of the surface expression of CD3 (T-lymphocyte) and CD19 (B-lymphocyte)/CD14 (monocyte) (B). Finally, CD3−/CD56+ cells were gated into 2 main subsets and 5 subsets depending on the expression of CD56 and CD16 (C). The numbers in panel (C) represent the following subsets: 1) CD56bright/CD16−, 2) CD56bright/CD16+, 3) CD56dim/CD16−, 4) CD56dim/CD16+, and 5) CD56−/CD16+.
|
NK cell cytotoxicity was assayed by evaluating the CD69 level in whole blood [
17]. To determine NK cell activation, heparinized whole blood (100 µL) was co-cultured with or without K562 tumor cells in 0.5 ml of complete RPMI-1640 medium for 2 h in an incubator-shaker and then 20 h at 37℃ in an incubator with a humidified 5% CO
2 atmosphere. The cells were mixed and centrifuged, and the supernatant was decanted and treated with FITC-, PE- and PECy5-conjugated monoclonal antibodies to CD3, CD56, and CD69 for 15 m. Next, red blood cells in whole blood samples were lysed for 10 m. The expression of CD69 on NK cells from the CD3
−/CD56
+ gate was analyzed.
The results were analyzed using SPSS software version 18.0.0 (SPSS Inc., Chicago, IL). Demographic and laboratory data were analyzed by the independent t-test or chi-squared test. The flow cytometry results were compared by the t-test, and are expressed as means ± standard deviation (SD). The associations of pain-related parameters with NK cell data were evaluated by Spearman's correlation analysis for NRS scores and the nonparametric Mann-Whitney U-test for disease or opioid use. All of the above tests were two-tailed, and a P value < 0.05 was considered indicative of statistical significance.
Go to :

RESULTS
This was a comparative, cross-sectional and non-experimental study involving a total of 60 subjects. Thirty participants had chronic moderate-to-severe pain (group P), and the remaining 30 did not (group NoP). As all of the subjects were age-matched, the mean age was not significantly different between the two groups. Moreover, the male/female ratio did not differ significantly between the two groups (
Table 1).
Table 1
Demographic and Pain-Related Data


The mean pain intensity (NRS score) in group P was 7.6, which could be categorized as severe pain, although the scores of 6 patients were < 7 [
1819]. The mean duration was 90 months, considerably longer than the cut-off for chronic pain (3 months). In group P, there were 6 patients with CRPS (I/II, 2/4), 5 with postherpetic neuralgia, 5 with post-spinal surgery syndrome, 2 with spinal cord injury pain, 10 with degenerative spinal disease, 1 with atypical facial pain, and 1 with painful diabetic neuropathy. Of the 30 subjects in group P, 24 had taken opioids (fentanyl, morphine, oxycodone, or tramadol). Four patients had undergone spinal cord stimulation, and one patient had received intrathecal morphine administration via a pump.
There was no significant difference in white blood cell count between the two groups. Flow cytometry analysis for lymphocytes revealed no significant differences in the percentage of NK cells in the peripheral blood of groups P and NoP (16.3 ± 9.2 vs. 20.2 ± 10.5%; not significant).
No significant difference in the percentage of the CD56
dim NK cell subset, which in the general population represents 90% of NK cells, was observed between group P and group NoP (88.8 ± 7.9 vs. 89.2 ± 7.6%). Furthermore, there was no significant difference in the percentage of the CD56
bright subset (< 10% in the general population) between group P and group NoP (2.1 ± 1.7 vs. 1.4 ± 1.4%; ns). The percentages of CD56
bright NK cells in both groups were within the reference range of the Korean population [
7]; further, group P had a higher percentage of CD56bright CD16
+ cells, a subset of CD56
bright, than did group NoP (1.0 ± 0.9 vs. 0.5 ± 0.6%;
P < 0.01) (
Fig. 2).
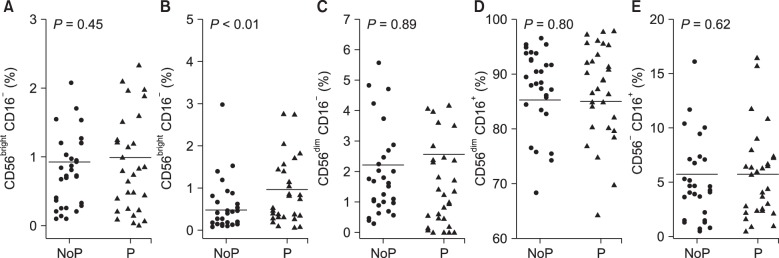 | Fig. 2Comparison of the percentages of 5 NK cell subsets in group NoP and P. The y-axes indicates the percentage of each NK cell subset, and each dot represents each value (percentage) of NK subsets in group NoP (•) and P (▴).
|
CD69 expression on NK cells did not differ significantly between group P and group NoP (29.2 ± 15.2 vs. 32.0 ± 15.0%; ns). In group P, subjects with CRPS showed a lower level of CD69 expression and CD56
bright/CD16
+cells than did subjects with other diseases, although the difference did not reach the level of statistical significance (
Table 2). Similarly, opioid users did not have a different NK cell profile including CD56
bright/CD16
+ and CD69 expression compared to the other subjects of group P. Although the intensity of pain also showed an inverse correlation with the percentage of CD56
bright/CD16
+ NK cells or CD69 expression, it was not significant (
Fig. 3).
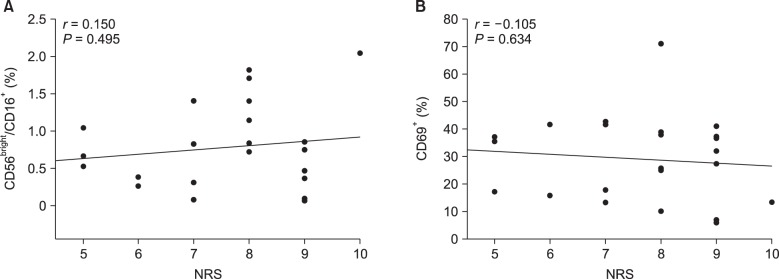 | Fig. 3Pain intensity (numerical rating scale, NRS) was not correlated with the increase in CD56bright/CD16+ cells (A) or percentage of cells expressing CD69 (B) ingroup P. Spearman's correlation test was used; r represents Spearman's coefficient.
|
Table 2
Percentage of CD56bright/CD16+ and CD69+ NK Cells in Group P


Go to :

DISCUSSION
This study shows that the CD56bright/CD16+ subset of NK cells was significantly increased in patients with chronic and moderate-to-severe pain, while the cytotoxic activity of NK cells and the abundances of the other 4 subsets did not differ from those in subjects without pain. In addition, the increase in the CD56bright/CD16+ subset or cytotoxicity of NK cells in pain patients was not correlated with the intensity of pain, opioid use, or the disease entity causing pain.
NK cells, representing 10–20% of lymphocytes in the peripheral blood, play a pivotal role in innate and adaptive immunity. Direct cytotoxicity and secretion of immunoregulatory cytokines and chemokines are the two main mechanisms of NK cell function. NK cells do not express CD3 or CD19, surface markers of T- and B-cells, respectively, but they express various levels of CD56 and CD16 depending on the subset type [
89]. Peripheral blood NK cells are divided into the two major subsets, CD56
dim and CD56
bright, depending on their level of CD56 expression. CD56
bright cells comprise approximately 10% of NK cells, proliferate upon activation, and produce a variety of cytokines and chemokines, but have minimal direct cytotoxic activity. In contrast, 90% of peripheral NK cells are of the CD56
dim subset and highly cytotoxic [
202122].
Despite the similar profiles of the two major NK cell subsets (CD56
bright and CD56
dim) in the two groups, a significant difference in the subset of CD56
bright/CD16
+ was observed in group P. Engagement of CD16 activates the cytotoxic function of NK cells by recognizing IgG bound to a broad range of targets [
20], but the main function of CD56
bright/CD16
+ is cytokine production. In addition, the percentage of CD56
bright/CD16
+ was approximately 1% of the total NK cells. Furthermore, the overall level of cytotoxic activity as evaluated by CD69 expression on NK cells was not significantly higher in group P than in group NoP. Therefore, the increased percentage of the CD56
bright/CD16
+ subset did not result in enhanced cytotoxic activity in group P.
Few studies have examined NK cell parameters in patients with back pain, fibromyalgia, and CRPS [
1213141516]. Consistent with our findings, a previous study found no significant difference in the percentages of T-, B- and NK cells in CRPS patients; however, NK cell activity was not assessed [
12]. In contrast, patients with chronic back pain had a lower percentage of NK cells [
13]. A study of female patients with fibromyalgia showed no attenuation of NK cell cytotoxic activity, but the patients had significantly fewer NK cells than healthy controls [
14]. In a study of patients with chronic pain, the percentage of NK cells in pain patients did not differ from that in patients without pain, but the NK cells showed a greater cytotoxic activity as assessed by the effector-to-target-cell ratios of NK cells and tumor cells [
15]. An intra-group analysis of this study also showed an inverse but non-significant association between cytotoxic activity and the Short-form McGill Pain Questionnaire (SF-MPQ) score. Similarly, patients with a higher NRS score tend to have lower CD69 expression, but this result did not reach statistical significance (
Fig. 3).
Patients with chronic pain require frequent administration of high doses of opioids for prolonged periods. Opioids exert an immunosuppressive effect [
23] by exerting direct and indirect effects on the activity of immune cells, including NK cells. The direct effects of opioids on immunity are mediated by the opioid receptor and their indirect effects by the sympathetic nervous system and HPA axis. Pain relief by opioids may ameliorate the immunosuppressive effect of pain. In addition, the effects of opioids on NK cells differ depending on the type of opioid and the clinical setting [
11]. Until now, the net clinical effect of opioid use has been unclear. In this study, opioid use did not affect NK cell cytotoxicity or subset populations in patients with chronic pain.
The unexpected results of this study may be due to the heterogeneous pain diseases in group P. There were patients with pure neuropathic pain such as post-herpetic neuralgia; however, some patients could be regarded as having complex nociceptive or inflammatory as well as neuropathic pain. One of the limitations of this study is that cytotoxicity was assessed indirectly by measuring the expression of CD69; a more direct measurement method may have been useful, such as direct monitoring of the death of K562 tumor cells. Interestingly, CD69 expression and CD56bright/CD16+ NK cells were lower in patients with CRPS, opioid use, or a higher NRS score, although the findings are not statistically significant. These results of our intra-group comparisons for group P could also have been influenced by the small number of enrolled subjects.
The clinical significance of the findings of both previous works and our study are unclear. However, it is likely that a certain group of patients should be in a state of immune suppression requiring a diagnostic workup or treatment. Additional well-designed studies that focus on multiple pain-related factors, including pain-causing disease, intensity and duration of pain, medications, or comorbidities such as depression, are needed to determine if immune function tests are necessary for patients with chronic and severe pain.
Our findings demonstrate that NK cell cytotoxic activity and the proportions of the major NK cell subsets, with the exception of an increased percentage of the CD56bright/CD16+ subset, are not significantly different between patients with chronic severe pain and pain free subjects. Moreover, pain-causing disease, opioid use, and pain intensity are not associated with NK cell cytotoxicity or subset populations.
Go to :






 PDF
PDF Citation
Citation Print
Print






 XML Download
XML Download